I am taking a bit of time to briefly discuss cloning. While I cannot guarantee the quality of my other intellectual posts, I will do my best to bring it into this last such post.
Dolly, a Finn Dorset sheep, was the first mammal to be cloned from an adult cell in 1996. Before then, a couple other animals were cloned. Since then, many other animals have been cloned. Among these animals are tadpoles, carp, cats, and horses. Some people consider cloning to be a patch on the problem of endangered species.
But what is cloning? It is the process of creating an identical copy of something. In organisms, it refers to creating copies of DNA or cells or organisms. In the case of certain organisms, it could also be asexual reproduction.
An example of cellular cloning would be creating a swatch of skin from a single cell. There are certain advantages to things such as this. Tissue cultures can be used in medicine. Skin, for example, could be used to help victims with severe burns. These cultures are rather difficult to cultivate, as they will not grow unless they are exposed to certain conditions.
We have genetically modified plants and animals. We have been cloning animals successfully since 1952. But there are two commonly accepted reasons for cloning. There is therapeutic cloning. This is done to produce various cells, for organs or various body parts. If technology advances to the point where this can be done with any sort of efficiency and certainty, it could lead to medical "magic." Transplant patients must take immunosuppressive drugs now. But if science can create exact copies of someone's organs would become unnecessary.
But there is also reproductive cloning. This is done solely for the purpose of creating copies of organisms. This is done be transferring material so that the created organism is a genetic copy of the donor. These copies are not completely exact, due to possible genetic mutations and mitochondrial genomes. It is this type of cloning which is most worrisome, especially when it comes to the prospect of human cloning. In 2002, the National Academy of Sciences called for a legally enforced ban on such cloning. The Academy, however, did support continued research on therapeutic cloning because of its possible medical benefits.
There are quite a few ethical issues with cloning as well. Horticultural cloning has been going on for centuries. But the problems come in with animal and human cloning. One argument against human cloning is that it should not be done, even to save the life an individual. The Christian argument is that life begins at conception and cloning is, in a sense, "playing God." Generally, Judaism is more accepting of cloning, as life is not equated with conception. Liberals protest cloning because of the perceived right of a person to protect his/her genetic material.
So, do we clone? Do we keep a complete ban on cloning? Your opinions.
~Interminable Immediacy
Monday, November 12, 2007
Wednesday, October 31, 2007
Happy Halloween!
 Happy Halloween, ladies and gentlemen readers! Instead of discussing cloning today, I have decided on a more timely topic. That's right, you can start groaning now. It's the history of Halloween. Why do I do this? It is important to know how our modern holidays developed. And what better holiday than this?
Happy Halloween, ladies and gentlemen readers! Instead of discussing cloning today, I have decided on a more timely topic. That's right, you can start groaning now. It's the history of Halloween. Why do I do this? It is important to know how our modern holidays developed. And what better holiday than this?Halloween has its origins in a holiday celebrated by the Celts of Ireland, the United Kingdom, and Northern France. That holiday is called Samhain (sau-wain), celebrated the day before their new year on November 1. The Celts believed that, on October 31, the barriers separating the world of the living from that of the dead thinned. The dead, then, were able to cross back into the living world for the night. These returned spirits caused trouble and ruined crops. But their presence made it easier for Druids, Celtic priests, to make predictions about the future. At night, the Druids built large bonfires. The people celebrated and burned crops and animals as offerings to the gods. They dressed in costumes, generally made from animal skins and attempted to tell each other's fortunes. When the festivities were over, they would relight their hearths from the sacred bonfires. It was believed that doing so would help protect them during the coming winter season.
Things changed somewhat when the Romans came into town in 43CE. During the time they ruled, two Roman holidays were combined with Samhain. They were Feralia, a day to celebrate the dead, and the day to honor Pomona, goddess of fruit and trees. One of the traditions believed to have eventually evolved from these incorporations is that of bobbing for apples. By the 800's, Christianity had swept it and begun exerting its influence. Pope Boniface IV named November 1 "All Saint's Day." It is believed that he might have been attempted to continue Samhain in a manner more acceptable to Christianity. Later, in the 1000's, November 2 was named "All Soul's Day." October 31 was known as "All Hallows Eve." Eventually, the words were slurred into "Halloween."
There are several symbols associated with Halloween. Most things dealing with Halloween revolve around death, magic, and mystical creatures. Common symbols involve skeletons, witches, ghost stories, bats, haunted houses, and black cats. There are many others. The traditional colors for the holiday are black and orange. Perhaps the most prominent symbol, however, is that of the Jack O'Lantern. The tradition of pumpkin carving derives from the Celtic myth of Stingy Jack.
To condense the story, Stingy Jack tricked the Devil twice. The first time, he tricked him into turning into a coin and kept him into his pocket next to a silver cross. When Jack released him, they made a deal. The Devil would leave him alone for a year and not take his soul upon death. At the end of the allotted time, Jack again tricked the Devil into another deal. Soon after, Jack died. God would not let him into Heaven and the Devil would not take his soul. With only a glowing coal, Jack was left to wander the earth. Legend says he put it into a carved-out turnip.
That, ladies and gentlemen, completes the entirety of useful information. Thank you for reading. Have a safe, happy Halloween and a blessed Samhain. Don't eat too much candy.
~Interminable Immediacy
Tuesday, October 30, 2007
Stem Cells
 Stem cells. We've all heard the arguments about them. But just what is so important about these things? Aren't they just cells like any other?
Stem cells. We've all heard the arguments about them. But just what is so important about these things? Aren't they just cells like any other?No, stem cells are not like every other bodily cell. A stem cell is "an unspecialized cell that gives rise to differentiated cells
"S
Stem cells, both adult and embryonic, have properties. The first property of stem cells is their ability to undergo several divisions while retaining their undefined state. The second is their potency. There are four levels of potency: totipotent, pluripotent, multipotent, and unipotent. Totipotent and pluripotent cells are the move variable of the four. Totipotent stem cells are formed when a sperm fertilizes an egg and the first few divisions. Pluripotent cells are slightly less variable, and can differentiate into cells derived from the germ layers (cells formed during embryogenesis, or formation). Multipotent cells can only produce cells within a certain family. For example, hematopoietic cells can differentiate into red and white blood cells and blood platelets. Finally, unipotent cells can only produce one type of cell but have a self-renewing property.
Stem cells have the potential to be extremely useful in the medical field. In fact, a number of adult stem cell treatments. A good example of these are bone marrow transplants, used to treat people afflicted by leukemia. But scientists and doctors hope to use stem cells to treat a wider variety of diseases, including Parkinson's Disease and muscle injuries. But there are a lot of issues surrounding the use of stem cells, issues which can be ironed out through debate and research.
There is quite a bit of controversy concerning stem cells and stem cell research. One of the main issues surrounding these cells is that starting a stem cell line requires the destruction of an embryo. That, or therapeutic cloning. However, there might be another way of creating embryonic stem cells. Opponents of stem cell research claim it to be another step toward reproductive cloning. Proponents cite the potential medical uses as reason enough to continue and expand stem cell research.
Do you, dear readers, have any thoughts on the matter? Based on the information provided, putting all moral issues aside, would you support stem cell research?
~Interminable Immediacy
Thursday, October 18, 2007
The End of an Age
Like the end of the oil-consuming era, the segment on energy is also coming to a close. Just to finish things up, I'm going to recap one or two things. (Not everything, as that would be terribly long and drawn out.)
Coal, Natural Gas, Oil - These are fossil fuels formed from organic matter buried within the ground. They provide sources of energy, but are also sources of CO2. While they are useful, it is essential that we move past these forms of energy to something cleaner and more efficient.
Nuclear - While potentially dangerous, nuclear power is a good backup. It provides constant energy, with little to no damage to the environment. Fission works right now, and fusion might at some point in the future. If nothing else, nuclear power is something to consider as a temporary replacement to oil and gas.
Hydrogen - While environmentally friendly, the technology for hydrogen isn't fully developed. If we can discover better methods of recovering elemental hydrogen, perhaps it might be a viable option. But that is millions of research dollars and years away.
Biofuel - One of the most controversial energy sources, in my mind. Renewable, to and extent. But production currently requires the burning of fossil fuels to produce the ethanol and bio diesel. And there's the issue of energy return: it's not 100%. Again, more research is needed. If we can improve the return ratio and reduce our reliance on fossil fuels to produce biofuel, it might just be one of the best options for the future.
What I have covered in these blogs are just the very tip of the iceberg, so to speak. There is so much more information out there. I encourage you to look further into the subject, to familiarize yourself with more of the scientific pros and cons of each topic. They are fascinating and absolutely essential to the continuation of our current way of life.
~Interminable Immediacy
Coal, Natural Gas, Oil - These are fossil fuels formed from organic matter buried within the ground. They provide sources of energy, but are also sources of CO2. While they are useful, it is essential that we move past these forms of energy to something cleaner and more efficient.
Nuclear - While potentially dangerous, nuclear power is a good backup. It provides constant energy, with little to no damage to the environment. Fission works right now, and fusion might at some point in the future. If nothing else, nuclear power is something to consider as a temporary replacement to oil and gas.
Hydrogen - While environmentally friendly, the technology for hydrogen isn't fully developed. If we can discover better methods of recovering elemental hydrogen, perhaps it might be a viable option. But that is millions of research dollars and years away.
Biofuel - One of the most controversial energy sources, in my mind. Renewable, to and extent. But production currently requires the burning of fossil fuels to produce the ethanol and bio diesel. And there's the issue of energy return: it's not 100%. Again, more research is needed. If we can improve the return ratio and reduce our reliance on fossil fuels to produce biofuel, it might just be one of the best options for the future.
What I have covered in these blogs are just the very tip of the iceberg, so to speak. There is so much more information out there. I encourage you to look further into the subject, to familiarize yourself with more of the scientific pros and cons of each topic. They are fascinating and absolutely essential to the continuation of our current way of life.
~Interminable Immediacy
Wednesday, October 17, 2007
The Aftermath
So what consequences will we have to deal with, as a result of our energy issues? The book The Long Emergency, by James Howard Kunstler, addresses some of them. For one thing, suburbia is dead. People will need to live in the cities, with easier access to jobs, public transportation, etc. That, or we will revert back to a more rural society, with most people living in small communities where they grow their own food. According to Kunstler:
“The dirty secret of the American economy in the 1990’s was that is was no longer about anything except the creation of suburban sprawl and the furnishing, accessorizing and the financing of it. It resembled the efficiency of cancer. Nothing else really mattered except building suburban houses, trading away the mortgages, selling the multiple cars needed by the inhabitants, upgrading the roads into commercial strip highways with all the necessary shopping infrastructure, and moving vast supplies of merchandise made in China for next to nothing to fill up those houses”
But there is a positive side. For example, those living in urban environments can bike or walk, instead of driving cars. And, wonder of wonders, it might just improve our health while we're at it. And city planning will become essential. As some people will inevitably move into the city, space must be economized. And it must be done in a manner that doesn't call for a lot of automotive traffic. The better planned things are, the more individuals can rely on more pedestrian forms of transportation. This, of course, is the concept of the "sustainable city."
Steven Wheeler put forth this definition of the sustainable city in 1998: "[the sustainably city is one where it has] development that improves the long-term social and ecological health of cities and towns." His ideas for this covered several topics, from economic land use and waste reduction to the restoration of natural environments. He also mentioned good living conditions, sustainable economics, community participation, and preservation of local culture. This is an ongoing process, and not an easy one. Urban areas have to be safe, with a lack of "slums," with available forms of non-polluting/minimal pollution transportation, opportunities for urban renewal, and aesthetics. All of these must also be considered in the long term, with opportunities for change and adaptation.
I realize this post is rather short, but it's basically just getting you to think. Suburban sprawl is unsustainable. As oil becomes more expensive, gas prices will rise. Consequently, it limits our transportation options. Any ideas on what can be done? Or how we can improve on urban and regional planning?
Steven Wheeler put forth this definition of the sustainable city in 1998: "[the sustainably city is one where it has] development that improves the long-term social and ecological health of cities and towns." His ideas for this covered several topics, from economic land use and waste reduction to the restoration of natural environments. He also mentioned good living conditions, sustainable economics, community participation, and preservation of local culture. This is an ongoing process, and not an easy one. Urban areas have to be safe, with a lack of "slums," with available forms of non-polluting/minimal pollution transportation, opportunities for urban renewal, and aesthetics. All of these must also be considered in the long term, with opportunities for change and adaptation.
I realize this post is rather short, but it's basically just getting you to think. Suburban sprawl is unsustainable. As oil becomes more expensive, gas prices will rise. Consequently, it limits our transportation options. Any ideas on what can be done? Or how we can improve on urban and regional planning?
Tuesday, October 9, 2007
Black Gold
 The one energy related issue I have not previously discussed is petroleum, gasoline, oil, black gold. Whatever you want to call it, it is the the main source of the problems we face today. Our economy is driven by oil (no pun intended). As reserves dwindle, we find ourselves on a slippery slope. Do we look the other way? Do we put time and resources into research? I have discussed the possible alternative energy sources. Now it's time to hit the crux of the matter.
The one energy related issue I have not previously discussed is petroleum, gasoline, oil, black gold. Whatever you want to call it, it is the the main source of the problems we face today. Our economy is driven by oil (no pun intended). As reserves dwindle, we find ourselves on a slippery slope. Do we look the other way? Do we put time and resources into research? I have discussed the possible alternative energy sources. Now it's time to hit the crux of the matter.As with the other fossil fuels, it is good to discuss the origins of oil. There are several conditions that need to be perfect or nearly perfect for oil to form:
1. Basins at the edges of the oceans must have a high concentration of organic material (5% or over) in their sedimentary rock. These conditions are extremely rare. If it weren't, there would be a lot more oil than there is currently.
2. The sediments have to be withing the oil window, 7500-15000ft. At 7500ft, the temperature causes large organic molecules to break down. Molecules with 5-20 carbon atoms are liquid at room temperature and pressure. Any less than 5 and the molecule is a gas at room temp and pressure.
3. Organic rick sediment buried below 15000ft will be "dry" natural gas, not oil.
4. About 90% of the oil finds its way to the surface via oil seeps. Only 10% gets trapped underground.
5. Porous rocks such as sandstone, limestone (CaCO3), and dolomite (CaMg(CO3)2) must be present to serve as reservoirs.
6. The pores in the host rock must be connected to each other, allowing for oil flow (permeability). The larger the pores in the rock, the more permeable it is. That being said, the permeability is the square of the grain size. Increasing the grain by 2 increases permeability by 4. And so on.
7. There must be a layer of rock above the oil reservoir that is relatively leak-proof. A few examples of such cap-stone rock types are fine-grained mudstone, halite (NaCl), and anhydrite (CaSO4).
Without even one of these factors, there will be no oil. The Middle East is prime oil country as the conditions there are just near perfect. As it stands, Saudi Arabia is the largest producer of oil in the world (7.7 million barrels/day with only 1600 working wells). The next largest produces are Russia (7.4 mil barrels/day, 41000 wells) and the United States (5.8 mil barrels/day, 521,000 wells).
 In the past, oil drilling was done by "banging a chisel up and down on the end of a rope." Eventually, drills were developed which operated using a heavy bit of wire with a drill bit attached to it. A thin mud flows back and forth in the drill pipe. However, other methods have been developed.
In the past, oil drilling was done by "banging a chisel up and down on the end of a rope." Eventually, drills were developed which operated using a heavy bit of wire with a drill bit attached to it. A thin mud flows back and forth in the drill pipe. However, other methods have been developed.If you recall, a recent commercial depicted a "man with a problem." In one of the commercials, he was sitting in a shop with his son when the boy bent his straw to get the last bit of his drink from the bottom and sides of his glass. I'm sorry to say, but horizontal drilling is not a new invention. It has been around for years. But it is effective. It can turn a vertical well into a horizontal well. This can be effective for up to a half mile or more. "Diamond bits" are tungsten-carbide drill heads in which synthetic diamonds are embedded. The hardness of the diamond allows drilling to be done much more quickly, and can drill up to 7000ft. It saves costs, too, since the drilling doesn't have to stop to replace worn our drill bits.
Recently Boeing Corporation donated several powerful lasers to the Colorado School of Mines. This was done in hopes of finding even faster drilling methods. (It should be noted the lasers were from the abandoned Star Wars program.) Finally, some rigs use continuous drilling, unrolling pipe like fishing line.
When the oil well is initially breached, the oil and gas comes bubbling up because the pressure is not equal. However, it eventually dies down and pumpjacks are set up to bring the oil to the surface. Once the easily accessible oil is retrieved, secondary recovery is started. Early on, this was accomplished by flooding the well with water so that the lighter oil could be recovered. Unfortunately, most efforts only recovered about half of the available oil in the reservoir. But other methods have been developed. Carbon dioxide is one especially good method. It is extremely soluble in crude oil and it gets the oil moving again. The drawback? 100% of the reservoir oil isn't recoverable no matter what we do.
We can't forget the rising price of oil, either. As oil becomes more scarce, the price rises to balance drilling and exploration costs against consumer demand. The Organization of the Petroleum Exporting Countries (OPEC) was formed, in part, to help regulate the price of oil. OPEC no longer controls the price of oil. But, then, no one does.
Most oil is used for transportation (a fact mentioned in previous posts). But something needs to be done. Technology that doesn't rely on oil. Better city planning. More people carpooling or using public transportation. Thoughts?
Monday, October 8, 2007
Biodiesel?
I find it amusing that, in the course of my discussion on energy, the newest National Geographic comes out. What is the topic of this month's issue? Global warming and biodiesel. I have already covered global warming; to do so again would be to beat it with a stick. Instead, I shall focus on this newest topic. Fuel produced from crops such as soy beans, corn, and sugar cane. "Proponents say such renewable fuels could light a fire under our moribund rural economy, help extract us from our sticky dependence on the Middle East, and–best of all–cut our ballooning emissions of carbon dioxide."
Biodiesel: the miracle replacement for gasoline. Some cars can run completely on ethanol or biodiesel. But here's one of the major issues. Compared to a gallon of gasoline, ethanol has only 67% of the energy content. Biodiesel is better, but it is still only 86% compared to a gallon of diesel. How do we reconcile this lack? Well, one way to do so is to look at the environmental aspect of things. Since organic-based fuels use carbon that is in the ground, it is not putting extra CO2 into the atmosphere. In a manner of speaking, with the right technology and fuel efficiency, cars could become carbon neutral. Unfortunately, at current technology levels, "producing corn ethanol consumes just about as much fossil fuel as the ethanol itself replaces." Using all our crops to produce "grown fuel" would only replace approximately 6% of our diesel and gasoline consumption.
Despite this apparent disappointment however, there is a bright star. Brazil, producing diesel from sugarcane, has managed to curtail its reliance upon imported oil. The United States government has pledged nearly $200 million to research, hoping to be able to replace up to 15% of our oil reliance by 2017. But the key, overall, is to produce oil from sources other than foodstuffs. If we can manage that, we will be better off. We will still have enough food to feed our burgeoning population as well as keep out livestock fatted.
 The original car models ran on alcohol, but it was expensive and didn't provide nearly as much energy as conventional refined petroleum. But that has changed some, since the introduction of ethanol-gasoline fuel mixes. Methyl tertiary-butyl ether (MTBE) was the additive used by oil companies for the same purpose. However, when it began to show up in aquifers (underground layers of water-bearing porous rock from which water can be extracted via wells), its use was banned. It didn't help that MTBE was believed to be carcinogenic (a cause of cancer).
The original car models ran on alcohol, but it was expensive and didn't provide nearly as much energy as conventional refined petroleum. But that has changed some, since the introduction of ethanol-gasoline fuel mixes. Methyl tertiary-butyl ether (MTBE) was the additive used by oil companies for the same purpose. However, when it began to show up in aquifers (underground layers of water-bearing porous rock from which water can be extracted via wells), its use was banned. It didn't help that MTBE was believed to be carcinogenic (a cause of cancer).
An extra benefit to the biodiesel/ethanol industry is the fact that it can jump-start small town economies. With farmers selling their crops, plants that produce these fuels create numerous job opportunities. The prices of corn and soybeans goes up, up to $4/bushel.
Again, there are issues. E85 (85% ethanol, 15% gasoline) "delivers 30 percent fewer miles a gallon than gasoline." And it can only be burned in specially designed engines. BUT! It is cheaper than regular gasoline. Its transport can be rather costly, but with plants springing up (ha!) all over the place, it should keep prices comparatively low.
Ethanol is alcohol. It is distilled through a process that hasn't much changed through the centuries. The grain is ground, then mixed with water and heated. Enzymes turn starch into sugar, then yeast is added. In the fermentation tanks, the yeast converts the sugar into alcohol. The alcohol is then separated from the water. What is left is fed to cows or spread over crops to be used as fertilizer. The drawback comes from the use of fossil fuels to heat the mixture, giving off carbon dioxide (which is also produced by the yeast). Some studies claim that ethanol is a losing battle, others make it to be more beneficial. Either way, it is not a cure-all solution. "Biofuels are a total waste and misleading us from getting at what we really need to do: conservation," says Cornell University's David Pimentel, who is one of ethanol's harshest critics. "This is a threat, not a service. Many people are seeing this as a boondoggle." However, proponents of ethanol, especially those who produce it, believe they can do things better. "They plan to fire their boilers with methane from two giant four-million-gallon biodigesters fed with cattle manure from the feedlot next door–in effect using biogas to make biofuel." (This amuses me, I should like to point out.)
Good and bad go hand-in-hand in the ethanol/biodiesel industry. But look again the the example of Brazil. When OPEC put an embargo on oil, Brazil turned to ethanol for fuel. It has done so again and most Brazilian cars haven't burned gasoline in years. Ethanol has a high octane rating (113) and burns better at higher compression. What is the secret to Brazilian success? Sugar cane! Yes, the same cane used to produce refined sugar for our tables. The plant is already %20 sugar and begins to ferment almost immediately after being cut, unlike corn which needs to convert starch to sugar. And it produces nearly twice as much ethanol as corn. The wastewater from the process, just like that from corn-based ethanol, can be used as fertilizer. And that is just how Brazilian producers use it. Another plus for the Brazilians is that they do not burn fossil fuels, but waste products. A final plus, researchers believe cane-based ethanol produces less carbon dioxide than gasoline (55-90%!) and the ethanol can be made from the stalks and leaves of the cane plant.
So, how do we respond? There are at least two other possible methods of creating biofuel: cellulose (from plant material) and algae (green algae, to be exact). There are pros and cons to each process, mainly in the department of research and development. However, the processes are out there and they are gaining notoriety and popularity. I say, if we can make biofuel work, let's go for it. Thoughts? Reactions?
Biodiesel: the miracle replacement for gasoline. Some cars can run completely on ethanol or biodiesel. But here's one of the major issues. Compared to a gallon of gasoline, ethanol has only 67% of the energy content. Biodiesel is better, but it is still only 86% compared to a gallon of diesel. How do we reconcile this lack? Well, one way to do so is to look at the environmental aspect of things. Since organic-based fuels use carbon that is in the ground, it is not putting extra CO2 into the atmosphere. In a manner of speaking, with the right technology and fuel efficiency, cars could become carbon neutral. Unfortunately, at current technology levels, "producing corn ethanol consumes just about as much fossil fuel as the ethanol itself replaces." Using all our crops to produce "grown fuel" would only replace approximately 6% of our diesel and gasoline consumption.
Despite this apparent disappointment however, there is a bright star. Brazil, producing diesel from sugarcane, has managed to curtail its reliance upon imported oil. The United States government has pledged nearly $200 million to research, hoping to be able to replace up to 15% of our oil reliance by 2017. But the key, overall, is to produce oil from sources other than foodstuffs. If we can manage that, we will be better off. We will still have enough food to feed our burgeoning population as well as keep out livestock fatted.
 The original car models ran on alcohol, but it was expensive and didn't provide nearly as much energy as conventional refined petroleum. But that has changed some, since the introduction of ethanol-gasoline fuel mixes. Methyl tertiary-butyl ether (MTBE) was the additive used by oil companies for the same purpose. However, when it began to show up in aquifers (underground layers of water-bearing porous rock from which water can be extracted via wells), its use was banned. It didn't help that MTBE was believed to be carcinogenic (a cause of cancer).
The original car models ran on alcohol, but it was expensive and didn't provide nearly as much energy as conventional refined petroleum. But that has changed some, since the introduction of ethanol-gasoline fuel mixes. Methyl tertiary-butyl ether (MTBE) was the additive used by oil companies for the same purpose. However, when it began to show up in aquifers (underground layers of water-bearing porous rock from which water can be extracted via wells), its use was banned. It didn't help that MTBE was believed to be carcinogenic (a cause of cancer).An extra benefit to the biodiesel/ethanol industry is the fact that it can jump-start small town economies. With farmers selling their crops, plants that produce these fuels create numerous job opportunities. The prices of corn and soybeans goes up, up to $4/bushel.
Again, there are issues. E85 (85% ethanol, 15% gasoline) "delivers 30 percent fewer miles a gallon than gasoline." And it can only be burned in specially designed engines. BUT! It is cheaper than regular gasoline. Its transport can be rather costly, but with plants springing up (ha!) all over the place, it should keep prices comparatively low.
Ethanol is alcohol. It is distilled through a process that hasn't much changed through the centuries. The grain is ground, then mixed with water and heated. Enzymes turn starch into sugar, then yeast is added. In the fermentation tanks, the yeast converts the sugar into alcohol. The alcohol is then separated from the water. What is left is fed to cows or spread over crops to be used as fertilizer. The drawback comes from the use of fossil fuels to heat the mixture, giving off carbon dioxide (which is also produced by the yeast). Some studies claim that ethanol is a losing battle, others make it to be more beneficial. Either way, it is not a cure-all solution. "Biofuels are a total waste and misleading us from getting at what we really need to do: conservation," says Cornell University's David Pimentel, who is one of ethanol's harshest critics. "This is a threat, not a service. Many people are seeing this as a boondoggle." However, proponents of ethanol, especially those who produce it, believe they can do things better. "They plan to fire their boilers with methane from two giant four-million-gallon biodigesters fed with cattle manure from the feedlot next door–in effect using biogas to make biofuel." (This amuses me, I should like to point out.)
Good and bad go hand-in-hand in the ethanol/biodiesel industry. But look again the the example of Brazil. When OPEC put an embargo on oil, Brazil turned to ethanol for fuel. It has done so again and most Brazilian cars haven't burned gasoline in years. Ethanol has a high octane rating (113) and burns better at higher compression. What is the secret to Brazilian success? Sugar cane! Yes, the same cane used to produce refined sugar for our tables. The plant is already %20 sugar and begins to ferment almost immediately after being cut, unlike corn which needs to convert starch to sugar. And it produces nearly twice as much ethanol as corn. The wastewater from the process, just like that from corn-based ethanol, can be used as fertilizer. And that is just how Brazilian producers use it. Another plus for the Brazilians is that they do not burn fossil fuels, but waste products. A final plus, researchers believe cane-based ethanol produces less carbon dioxide than gasoline (55-90%!) and the ethanol can be made from the stalks and leaves of the cane plant.
So, how do we respond? There are at least two other possible methods of creating biofuel: cellulose (from plant material) and algae (green algae, to be exact). There are pros and cons to each process, mainly in the department of research and development. However, the processes are out there and they are gaining notoriety and popularity. I say, if we can make biofuel work, let's go for it. Thoughts? Reactions?
Labels:
biodiesel,
energy,
environment,
ethanol,
power
Thursday, October 4, 2007
Hydrogen Miracle?
It has been speculated that hydrogen power might be the miracle cure for the world's energy problems. After all, it is the most abundant element. And the knowledge for using electricity to break apart water has been around since at least 1805, over two hundred years. So why do we still rely on fossil fuels?  The answer is simple: The miracle cure might not be so miraculous. There is a limit to the achievements of the hydrogen economy. What is the hydrogen economy? To put it simply, it is the hypothetical situation where automotive power is derived from reacting hydrogen with oxygen. The purpose of the hydrogen economy is to reduce carbon dioxide emissions from carbon-based fuels and to provide a replacement for dwindling petroleum reserves. This would make it a storage tool as opposed to nuclear fusion as a primary energy source.
The answer is simple: The miracle cure might not be so miraculous. There is a limit to the achievements of the hydrogen economy. What is the hydrogen economy? To put it simply, it is the hypothetical situation where automotive power is derived from reacting hydrogen with oxygen. The purpose of the hydrogen economy is to reduce carbon dioxide emissions from carbon-based fuels and to provide a replacement for dwindling petroleum reserves. This would make it a storage tool as opposed to nuclear fusion as a primary energy source.
We have to ask ourselves two questions, claims Kenneth Deffeyes. "Is hydrogen an effective solution to the problem?" and "Can we make an orderly transition from our present gasoline powered cars to a hydrogen fleet?" These can lead to two interesting problems. The first being a situation similar to that of ethanol. Ethanol, or corn oil, requires more energy input than that which is derived from the final product. If this is the case with hydrogen, then perhaps it is not so economical to utilize it as a fuel source.
The second issue is like that of natural gas powered vehicles. Is it a chicken and egg situation? Iceland has opened a hydrogen fueling station, but it is the only country to do so. Will other countries follow suit or will they wait until hydrogen cars are built? Will hydrogen cars be built if there are no filling stations? Governments can help the immediate situation by adding incentives if companies start producing and providing the necessary resources.
The biggest attraction of hydrogen energy is mobility. A large portion of petroleum is used for transportation. If we run out of petroleum, we lose a lot of transportation. But hydrogen can be used for a multitude of things. For example, according to a PBS article, Neah Power Systems in Seattle has developed a hydrogen battery which can provide power to laptops for up to 8 hours. If the technology advances, it could become efficient and powerful enough to power computers for far longer, or even to power automobiles. But most hydrogen today is used in making fertilizer and upgrading petroleum in refineries.
There are three ways of producing hydrogen known today. The first is known as the water-gas process. Put simply, it reacts methane (CH4) and steam (H2O) with a nickel catalyst at temperatures of 1500 °F. The end products are carbon dioxide (CO2) and hydrogen (H2).
 The answer is simple: The miracle cure might not be so miraculous. There is a limit to the achievements of the hydrogen economy. What is the hydrogen economy? To put it simply, it is the hypothetical situation where automotive power is derived from reacting hydrogen with oxygen. The purpose of the hydrogen economy is to reduce carbon dioxide emissions from carbon-based fuels and to provide a replacement for dwindling petroleum reserves. This would make it a storage tool as opposed to nuclear fusion as a primary energy source.
The answer is simple: The miracle cure might not be so miraculous. There is a limit to the achievements of the hydrogen economy. What is the hydrogen economy? To put it simply, it is the hypothetical situation where automotive power is derived from reacting hydrogen with oxygen. The purpose of the hydrogen economy is to reduce carbon dioxide emissions from carbon-based fuels and to provide a replacement for dwindling petroleum reserves. This would make it a storage tool as opposed to nuclear fusion as a primary energy source.We have to ask ourselves two questions, claims Kenneth Deffeyes. "Is hydrogen an effective solution to the problem?" and "Can we make an orderly transition from our present gasoline powered cars to a hydrogen fleet?" These can lead to two interesting problems. The first being a situation similar to that of ethanol. Ethanol, or corn oil, requires more energy input than that which is derived from the final product. If this is the case with hydrogen, then perhaps it is not so economical to utilize it as a fuel source.
The second issue is like that of natural gas powered vehicles. Is it a chicken and egg situation? Iceland has opened a hydrogen fueling station, but it is the only country to do so. Will other countries follow suit or will they wait until hydrogen cars are built? Will hydrogen cars be built if there are no filling stations? Governments can help the immediate situation by adding incentives if companies start producing and providing the necessary resources.
The biggest attraction of hydrogen energy is mobility. A large portion of petroleum is used for transportation. If we run out of petroleum, we lose a lot of transportation. But hydrogen can be used for a multitude of things. For example, according to a PBS article, Neah Power Systems in Seattle has developed a hydrogen battery which can provide power to laptops for up to 8 hours. If the technology advances, it could become efficient and powerful enough to power computers for far longer, or even to power automobiles. But most hydrogen today is used in making fertilizer and upgrading petroleum in refineries.
There are three ways of producing hydrogen known today. The first is known as the water-gas process. Put simply, it reacts methane (CH4) and steam (H2O) with a nickel catalyst at temperatures of 1500 °F. The end products are carbon dioxide (CO2) and hydrogen (H2).
CH4 + 2 H2O = CO2 + 4 H2
Natural gas is the easiest way to produce this hydrogen, being composed mainly of methane. However, in the absence of natural gas, coal is another option. If natural gas is used, it would be an economic cycle. The natural gas from oil wells could be used to produce hydrogen. Then the carbon dioxide waste could be used to recover more oil.
 The second process is electrolysis. This method is accomplished by using electrolytic cells. These are cell which contain a cathode (positive) and anode (negative), using electrical voltage to separate ions. As with anything, however, there is a positive and negative side to producing hydrogen in this manner. The positive is that current (in amperes) produces hydrogen with over 98% efficiency. The downside, however, is that the voltage required is 20-30% greater than the ideal
The second process is electrolysis. This method is accomplished by using electrolytic cells. These are cell which contain a cathode (positive) and anode (negative), using electrical voltage to separate ions. As with anything, however, there is a positive and negative side to producing hydrogen in this manner. The positive is that current (in amperes) produces hydrogen with over 98% efficiency. The downside, however, is that the voltage required is 20-30% greater than the ideal
What does this mean? Here's an example provided by Deffeyes in Beyond Oil. The most efficient commercial cells require 1.75-2.00 volts to produce hydrogen. The fuel cell only returns 0.7 volt. what does this mean? You only get back 40% of your overall volt input. But certainly we can develop a more efficient process! Theoretically, we can increase the efficiency by 30%, but the technology has stagnated. At the same time, solar and wind power can be used to produce hydrogen. However, these processes are not yet enough to compete with commercial electrolysis cells.
Finally, there are exotic hydrogen sources. Breaking down compounds which contain hydrogen works, but isn't done commercially. But the most interesting exotic source is purple bacteria. Instead of green chlorophyll, they have a different chemical compound which absorbs sunlight. Nothing is certain as of yet, but research in being conducted.
In the words of Nobel Prize-winning physicist Richard Smalley, "I believe it is the single most important problem facing humanity today: Energy. How are we going to get prosperous when oil and gas and coal are no longer enough?" The only way we can continue to prosper is by finding alternate sources of energy. Hydrogen is looking good, but there are definitely issues. If hydrogen power is to be completely non-polluting, the resources and methods used to produce it must also be non-polluting. There are also economic concerns about price. The final issues are, of course, storage and safety.
Hydrogen can be safely stored either as a pressurized gas or a cold liquid. Pressurized gas is stable, but large amounts of money are required to produce the necessary power. Liquid hydrogen, on the other hand, would require an insulated fuel cell and an escape route. With no escape route, the results could be... explosive. At current technological levels, liquid hydrogen is more efficient for consumption equivalent to 10 gallons of gasoline or greater. The major safety issue with hydrogen is that is burns. On its own, hydrogen gas isn't toxic if there is sufficient oxygen. But solutions of 4-75% hydrogen will burn. Hydrogen flames are nearly invisible and propagate at a rate of nearly 10ft/sec. Hydrogen can also undergo combustion in mixtures between 18-60% hydrogen. That mans absolutely no open flames near a hydrogen source unless it is very carefully controlled.
Overall, hydrogen seems like a good solution. But there are plenty of issues that need to be overcome before it is economically and commercially viable. So, what do we do? What are your thoughts on the matter?
 The second process is electrolysis. This method is accomplished by using electrolytic cells. These are cell which contain a cathode (positive) and anode (negative), using electrical voltage to separate ions. As with anything, however, there is a positive and negative side to producing hydrogen in this manner. The positive is that current (in amperes) produces hydrogen with over 98% efficiency. The downside, however, is that the voltage required is 20-30% greater than the ideal
The second process is electrolysis. This method is accomplished by using electrolytic cells. These are cell which contain a cathode (positive) and anode (negative), using electrical voltage to separate ions. As with anything, however, there is a positive and negative side to producing hydrogen in this manner. The positive is that current (in amperes) produces hydrogen with over 98% efficiency. The downside, however, is that the voltage required is 20-30% greater than the idealWhat does this mean? Here's an example provided by Deffeyes in Beyond Oil. The most efficient commercial cells require 1.75-2.00 volts to produce hydrogen. The fuel cell only returns 0.7 volt. what does this mean? You only get back 40% of your overall volt input. But certainly we can develop a more efficient process! Theoretically, we can increase the efficiency by 30%, but the technology has stagnated. At the same time, solar and wind power can be used to produce hydrogen. However, these processes are not yet enough to compete with commercial electrolysis cells.
Finally, there are exotic hydrogen sources. Breaking down compounds which contain hydrogen works, but isn't done commercially. But the most interesting exotic source is purple bacteria. Instead of green chlorophyll, they have a different chemical compound which absorbs sunlight. Nothing is certain as of yet, but research in being conducted.
In the words of Nobel Prize-winning physicist Richard Smalley, "I believe it is the single most important problem facing humanity today: Energy. How are we going to get prosperous when oil and gas and coal are no longer enough?" The only way we can continue to prosper is by finding alternate sources of energy. Hydrogen is looking good, but there are definitely issues. If hydrogen power is to be completely non-polluting, the resources and methods used to produce it must also be non-polluting. There are also economic concerns about price. The final issues are, of course, storage and safety.
Hydrogen can be safely stored either as a pressurized gas or a cold liquid. Pressurized gas is stable, but large amounts of money are required to produce the necessary power. Liquid hydrogen, on the other hand, would require an insulated fuel cell and an escape route. With no escape route, the results could be... explosive. At current technological levels, liquid hydrogen is more efficient for consumption equivalent to 10 gallons of gasoline or greater. The major safety issue with hydrogen is that is burns. On its own, hydrogen gas isn't toxic if there is sufficient oxygen. But solutions of 4-75% hydrogen will burn. Hydrogen flames are nearly invisible and propagate at a rate of nearly 10ft/sec. Hydrogen can also undergo combustion in mixtures between 18-60% hydrogen. That mans absolutely no open flames near a hydrogen source unless it is very carefully controlled.
Overall, hydrogen seems like a good solution. But there are plenty of issues that need to be overcome before it is economically and commercially viable. So, what do we do? What are your thoughts on the matter?
Tuesday, October 2, 2007
Is it hot in here? Global warming's to blame!
One of the major concerns with fossil fuel energy is the emission of greenhouse gases. The issue with greenhouse gases is called global warming. It is a hotly contested issue. My goal is to discuss some of concerns, theories, and possible solutions to this problem.
Global warming is the increase in the average temperature of the Earth's near-surface air and oceans, both in recent decades and its projected continuity. In the last 100 years or so, the temperature has risen approximately 1.33 ± 0.32 °F. There is a high probability that a main factor for this increase is due to human activity, mainly the release of greenhouse gases into the atmosphere. Before 1950, volcanoes and solar variance might have played a minor role in warming the Earth. Post 1950, it is believed they may have had a cooling effect. The Intergovernmental Panel on Climate Change (IPCC) has studied models which predict a rise in temperature ranging from 2.0 - 11.5 °F between 1990 and 2100.
 Greenhouse gases, the culprits behind global warming, are divided into four categories. They are carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and fluorinated gases. Carbon dioxide, methane, and nitrous oxide make up the largest segment of greenhouse gases in the atmosphere. A large percentage of these gases come from the burning of fossil fuels, the decomposition of matter, and other sources. These gases exist naturally. However, dangerous as they are, these are not the most powerful greenhouse gases. The fluorinated gases, like hydrofluorocarbons and perfluorocarbons, are powerful synthetic gases considered to be high global warming potential (GWP) gases. They are released during a number of industrial processes, such as the production of aluminum and magnesium, semiconductor manufacturing, and electric power transmission. In recent years, they have been used to replace various gases that deplete the ozone layer, like CFC's. Thankfully, despite their potency, they are released only in minor quantities.
Greenhouse gases, the culprits behind global warming, are divided into four categories. They are carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and fluorinated gases. Carbon dioxide, methane, and nitrous oxide make up the largest segment of greenhouse gases in the atmosphere. A large percentage of these gases come from the burning of fossil fuels, the decomposition of matter, and other sources. These gases exist naturally. However, dangerous as they are, these are not the most powerful greenhouse gases. The fluorinated gases, like hydrofluorocarbons and perfluorocarbons, are powerful synthetic gases considered to be high global warming potential (GWP) gases. They are released during a number of industrial processes, such as the production of aluminum and magnesium, semiconductor manufacturing, and electric power transmission. In recent years, they have been used to replace various gases that deplete the ozone layer, like CFC's. Thankfully, despite their potency, they are released only in minor quantities. Major industrial countries burning coal for power tend to produce more greenhouse gases than less industrialized countries. However, there are several theories concerning the buildup of these gases, as well as theories concerning the warming of the Earth. One of the major theories is that the warming of the planet is completely natural, since we are coming out of the "Little Ice Age." Proponents of this theory believe the warming is a part of the natural temperature variation, that is has an established trend, and need not be explained by outside sources. While some of this might be true, the existence of greenhouse gases in the atmosphere cannot be ignored.
Major industrial countries burning coal for power tend to produce more greenhouse gases than less industrialized countries. However, there are several theories concerning the buildup of these gases, as well as theories concerning the warming of the Earth. One of the major theories is that the warming of the planet is completely natural, since we are coming out of the "Little Ice Age." Proponents of this theory believe the warming is a part of the natural temperature variation, that is has an established trend, and need not be explained by outside sources. While some of this might be true, the existence of greenhouse gases in the atmosphere cannot be ignored.
 Another theory concerning global warming is based on deforestation. As any botanist knows, trees and other plants utilize CO2 as a part of their respiration. A byproduct of this process is oxygen, which is released back into the atmosphere. The process of photosynthesis carried out by plants is essential to reducing the CO2 concentration. However, with more and more forest being cleared for farmland, especially in the tropics, there are fewer trees to leech carbon dioxide from the atmosphere. This reduction in the number of trees is thought to have led, in part, to the increased concentration of carbon dioxide. It should be noted, as well, that the oceans play a part in removing CO2 by absorbing it. The process is balanced between natural CO2 production and the amount of the gas the ocean can absorb. But with humans pumping large amounts of greenhouse gases into the atmosphere, the oceans cannot keep up with the increased percentage.
Another theory concerning global warming is based on deforestation. As any botanist knows, trees and other plants utilize CO2 as a part of their respiration. A byproduct of this process is oxygen, which is released back into the atmosphere. The process of photosynthesis carried out by plants is essential to reducing the CO2 concentration. However, with more and more forest being cleared for farmland, especially in the tropics, there are fewer trees to leech carbon dioxide from the atmosphere. This reduction in the number of trees is thought to have led, in part, to the increased concentration of carbon dioxide. It should be noted, as well, that the oceans play a part in removing CO2 by absorbing it. The process is balanced between natural CO2 production and the amount of the gas the ocean can absorb. But with humans pumping large amounts of greenhouse gases into the atmosphere, the oceans cannot keep up with the increased percentage.
And yet, in some ways, global warming can be positive. Oddly enough, parts of the northern hemisphere have shown a certain amount of increased productivity. Despite this, the productivity is believe to be of finite proportions. Another possible benefit could be the emergence of the fabled Northwest Passage, which could cut thousands of nautical miles off voyages from Europe to Asia. However, the detriments appear to largely overpower the few possible benefits. For example, the rise in temperature is affecting various ecosystems. In the affected ecosystems, animal habitats are also being altered. At some point, these habitats may changed sufficiently that they are no longer suitable to the organisms which reside there. In that case, they will either die out or be forced to migrate to habitats more similar to their original one. One final possible effect of global warming is the spread of disease. It has been hypothesized that the increased temperatures have been expedient to the spread of diseases. At this point, though, that is just speculation as no-one is certain that this is true.
Finally, what can be done to help the situation? Some countries have put a tax on carbon, hoping to prompt companies to utilize less of the element, thereby producing less CO2. Other legal actions have been taken to reduce the output of greenhouse gases into the atmosphere. For example, in the United States, regulation of gas emissions have been given to the Environmental Protection Agency (EPA), under the Clean Air Act. The Clean Air Act is designed to help reduce air pollution. One way to achieve this is the use of renewable energy sources, such as wind, hyrdo, and solar power. While not available in all areas, wind and hydroelectric power plants can help. With more efficient absorption and storage, solar power can be extremely economical. Problems might occur on cloudy days, but those can be bypassed by storing electrical power.
The use of Energy Star appliances and turning them off while they're not in use can also reduce the production of greenhouse gases. This is a rather roundabout method, as the less electricity used mean less power needs to be generated. The purchase and use of more efficient, less polluting cars is another way to reduce emissions. Better sources of energy and transport will be developed in the future, but this is the now. Do you have any other suggestions or comments?
(For articles concerning global warming check out the New York Times science page.)
Global warming is the increase in the average temperature of the Earth's near-surface air and oceans, both in recent decades and its projected continuity. In the last 100 years or so, the temperature has risen approximately 1.33 ± 0.32 °F. There is a high probability that a main factor for this increase is due to human activity, mainly the release of greenhouse gases into the atmosphere. Before 1950, volcanoes and solar variance might have played a minor role in warming the Earth. Post 1950, it is believed they may have had a cooling effect. The Intergovernmental Panel on Climate Change (IPCC) has studied models which predict a rise in temperature ranging from 2.0 - 11.5 °F between 1990 and 2100.
 Greenhouse gases, the culprits behind global warming, are divided into four categories. They are carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and fluorinated gases. Carbon dioxide, methane, and nitrous oxide make up the largest segment of greenhouse gases in the atmosphere. A large percentage of these gases come from the burning of fossil fuels, the decomposition of matter, and other sources. These gases exist naturally. However, dangerous as they are, these are not the most powerful greenhouse gases. The fluorinated gases, like hydrofluorocarbons and perfluorocarbons, are powerful synthetic gases considered to be high global warming potential (GWP) gases. They are released during a number of industrial processes, such as the production of aluminum and magnesium, semiconductor manufacturing, and electric power transmission. In recent years, they have been used to replace various gases that deplete the ozone layer, like CFC's. Thankfully, despite their potency, they are released only in minor quantities.
Greenhouse gases, the culprits behind global warming, are divided into four categories. They are carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and fluorinated gases. Carbon dioxide, methane, and nitrous oxide make up the largest segment of greenhouse gases in the atmosphere. A large percentage of these gases come from the burning of fossil fuels, the decomposition of matter, and other sources. These gases exist naturally. However, dangerous as they are, these are not the most powerful greenhouse gases. The fluorinated gases, like hydrofluorocarbons and perfluorocarbons, are powerful synthetic gases considered to be high global warming potential (GWP) gases. They are released during a number of industrial processes, such as the production of aluminum and magnesium, semiconductor manufacturing, and electric power transmission. In recent years, they have been used to replace various gases that deplete the ozone layer, like CFC's. Thankfully, despite their potency, they are released only in minor quantities. Major industrial countries burning coal for power tend to produce more greenhouse gases than less industrialized countries. However, there are several theories concerning the buildup of these gases, as well as theories concerning the warming of the Earth. One of the major theories is that the warming of the planet is completely natural, since we are coming out of the "Little Ice Age." Proponents of this theory believe the warming is a part of the natural temperature variation, that is has an established trend, and need not be explained by outside sources. While some of this might be true, the existence of greenhouse gases in the atmosphere cannot be ignored.
Major industrial countries burning coal for power tend to produce more greenhouse gases than less industrialized countries. However, there are several theories concerning the buildup of these gases, as well as theories concerning the warming of the Earth. One of the major theories is that the warming of the planet is completely natural, since we are coming out of the "Little Ice Age." Proponents of this theory believe the warming is a part of the natural temperature variation, that is has an established trend, and need not be explained by outside sources. While some of this might be true, the existence of greenhouse gases in the atmosphere cannot be ignored. Another theory concerning global warming is based on deforestation. As any botanist knows, trees and other plants utilize CO2 as a part of their respiration. A byproduct of this process is oxygen, which is released back into the atmosphere. The process of photosynthesis carried out by plants is essential to reducing the CO2 concentration. However, with more and more forest being cleared for farmland, especially in the tropics, there are fewer trees to leech carbon dioxide from the atmosphere. This reduction in the number of trees is thought to have led, in part, to the increased concentration of carbon dioxide. It should be noted, as well, that the oceans play a part in removing CO2 by absorbing it. The process is balanced between natural CO2 production and the amount of the gas the ocean can absorb. But with humans pumping large amounts of greenhouse gases into the atmosphere, the oceans cannot keep up with the increased percentage.
Another theory concerning global warming is based on deforestation. As any botanist knows, trees and other plants utilize CO2 as a part of their respiration. A byproduct of this process is oxygen, which is released back into the atmosphere. The process of photosynthesis carried out by plants is essential to reducing the CO2 concentration. However, with more and more forest being cleared for farmland, especially in the tropics, there are fewer trees to leech carbon dioxide from the atmosphere. This reduction in the number of trees is thought to have led, in part, to the increased concentration of carbon dioxide. It should be noted, as well, that the oceans play a part in removing CO2 by absorbing it. The process is balanced between natural CO2 production and the amount of the gas the ocean can absorb. But with humans pumping large amounts of greenhouse gases into the atmosphere, the oceans cannot keep up with the increased percentage.And yet, in some ways, global warming can be positive. Oddly enough, parts of the northern hemisphere have shown a certain amount of increased productivity. Despite this, the productivity is believe to be of finite proportions. Another possible benefit could be the emergence of the fabled Northwest Passage, which could cut thousands of nautical miles off voyages from Europe to Asia. However, the detriments appear to largely overpower the few possible benefits. For example, the rise in temperature is affecting various ecosystems. In the affected ecosystems, animal habitats are also being altered. At some point, these habitats may changed sufficiently that they are no longer suitable to the organisms which reside there. In that case, they will either die out or be forced to migrate to habitats more similar to their original one. One final possible effect of global warming is the spread of disease. It has been hypothesized that the increased temperatures have been expedient to the spread of diseases. At this point, though, that is just speculation as no-one is certain that this is true.
Finally, what can be done to help the situation? Some countries have put a tax on carbon, hoping to prompt companies to utilize less of the element, thereby producing less CO2. Other legal actions have been taken to reduce the output of greenhouse gases into the atmosphere. For example, in the United States, regulation of gas emissions have been given to the Environmental Protection Agency (EPA), under the Clean Air Act. The Clean Air Act is designed to help reduce air pollution. One way to achieve this is the use of renewable energy sources, such as wind, hyrdo, and solar power. While not available in all areas, wind and hydroelectric power plants can help. With more efficient absorption and storage, solar power can be extremely economical. Problems might occur on cloudy days, but those can be bypassed by storing electrical power.
The use of Energy Star appliances and turning them off while they're not in use can also reduce the production of greenhouse gases. This is a rather roundabout method, as the less electricity used mean less power needs to be generated. The purchase and use of more efficient, less polluting cars is another way to reduce emissions. Better sources of energy and transport will be developed in the future, but this is the now. Do you have any other suggestions or comments?
(For articles concerning global warming check out the New York Times science page.)
Labels:
energy,
environment,
global warming,
power
Wednesday, September 26, 2007
Black Power: The Force of Coal
In the third installment of the power series, the topic is coal. I will briefly describe the origin of coal and a couple common types. I shall then go into the uses of coal. Finally, I will discuss the problems associated with the material. As with the discussion of natural gas, my main source for this article is the book Beyond Oil.
 (Coal)Coal, like other fossil fuels, is formed from organic materials. However, unlike oil and gas, which form from the decomposition of said materials, coal is formed in an entirely different manner. Coal was formed mainly during was has been termed the "Carboniferous period," which was approximately 280-310 million years ago. However, there are coal beds that are both younger and older than the Carboniferous period.
(Coal)Coal, like other fossil fuels, is formed from organic materials. However, unlike oil and gas, which form from the decomposition of said materials, coal is formed in an entirely different manner. Coal was formed mainly during was has been termed the "Carboniferous period," which was approximately 280-310 million years ago. However, there are coal beds that are both younger and older than the Carboniferous period.
Basically, the period was a cycle of flooding as the glaciers would melt and the sea level would rise. The rise in sea level would bury bogs and swamps, areas of significant botanical accumulation. Layers of soil and fossils would be deposited on top of the area and "baked" (for lack of a better term). The materials used in coal production, not just the temperature, helped determine the type of coal that resulted. For example, spores and pollen produce a type of coal that burns easily and leaves very little ash (cannel coal). Jet, on the other hand, is a product of wood, and is usually glossy black. It can be used in jewelry.
 Coal is further categorized by age. It ranges from liginte (jet) to bituminous to anthracite. Each new category has a greater percentage of carbon. Anthracite, the last of the line, is over 90% carbon. It can be ignited for use in industrial heating. The most commonly used coal, however, is bituminous. It is used both for producing electricity and something called coke. Coke is treated coal burned in large ovens to produce charred lumps.
Coal is further categorized by age. It ranges from liginte (jet) to bituminous to anthracite. Each new category has a greater percentage of carbon. Anthracite, the last of the line, is over 90% carbon. It can be ignited for use in industrial heating. The most commonly used coal, however, is bituminous. It is used both for producing electricity and something called coke. Coke is treated coal burned in large ovens to produce charred lumps.
The byproducts of coke can be used for various industries. For example, the gases can be burned for light and heat. Others of the gases can be used as catalysts for other reactions which produce iron from ore. "Coal oils" (hydrocarbons) can be used in lamps. Most interestingly, the tars produced have lead to vibrant synthetic dyes.
The main use for coal, however, is in the generation of electricity. Coal is burned to heat water. The steam created is then used to turn turbines which create electric current. However, this is a major problem with the use of coal. Most coal is impure, containing amounts of sulfur and mercury. Since coal is a hard material, it is difficult to remove the impurities in that form. And while the smoke can be scrubbed, but it is still difficult. Since the main byproduct of burned coal is CO2, that creates a problem with "greenhouse gases." Despite these problems, there are a lot of coal burning plants in the United States. Another major issue with coal burning is sulfur dioxide (SO2). In the atmosphere, sulfur dioxide becomes sulfuric acid. That, in turn, becomes a contributor to acid rain and smog. Other coal issues include mercury pollution, underground mind drainage, and major surface disruptions caused by open-pit mines.
 Despite this, coal is the best source of energy. It is the cheapest per unit of energy. It is also quite an industry. The US is one of the major producers of coal worldwide. There are over 6000 coal mines in the US alone. The former Soviet Union is also a major producer of coal. And coal reserves are large enough that, at the rate it is being mined, production should be able to continue for a few hundred years.
Despite this, coal is the best source of energy. It is the cheapest per unit of energy. It is also quite an industry. The US is one of the major producers of coal worldwide. There are over 6000 coal mines in the US alone. The former Soviet Union is also a major producer of coal. And coal reserves are large enough that, at the rate it is being mined, production should be able to continue for a few hundred years.
So, the problem is, do we continue using coal? Do we continue burning coal, producing greenhouse gases and acid rain? Or do we look for alternate sources of energy, which is inevitable anyway?
 (Coal)
(Coal)Basically, the period was a cycle of flooding as the glaciers would melt and the sea level would rise. The rise in sea level would bury bogs and swamps, areas of significant botanical accumulation. Layers of soil and fossils would be deposited on top of the area and "baked" (for lack of a better term). The materials used in coal production, not just the temperature, helped determine the type of coal that resulted. For example, spores and pollen produce a type of coal that burns easily and leaves very little ash (cannel coal). Jet, on the other hand, is a product of wood, and is usually glossy black. It can be used in jewelry.
 Coal is further categorized by age. It ranges from liginte (jet) to bituminous to anthracite. Each new category has a greater percentage of carbon. Anthracite, the last of the line, is over 90% carbon. It can be ignited for use in industrial heating. The most commonly used coal, however, is bituminous. It is used both for producing electricity and something called coke. Coke is treated coal burned in large ovens to produce charred lumps.
Coal is further categorized by age. It ranges from liginte (jet) to bituminous to anthracite. Each new category has a greater percentage of carbon. Anthracite, the last of the line, is over 90% carbon. It can be ignited for use in industrial heating. The most commonly used coal, however, is bituminous. It is used both for producing electricity and something called coke. Coke is treated coal burned in large ovens to produce charred lumps.The byproducts of coke can be used for various industries. For example, the gases can be burned for light and heat. Others of the gases can be used as catalysts for other reactions which produce iron from ore. "Coal oils" (hydrocarbons) can be used in lamps. Most interestingly, the tars produced have lead to vibrant synthetic dyes.
The main use for coal, however, is in the generation of electricity. Coal is burned to heat water. The steam created is then used to turn turbines which create electric current. However, this is a major problem with the use of coal. Most coal is impure, containing amounts of sulfur and mercury. Since coal is a hard material, it is difficult to remove the impurities in that form. And while the smoke can be scrubbed, but it is still difficult. Since the main byproduct of burned coal is CO2, that creates a problem with "greenhouse gases." Despite these problems, there are a lot of coal burning plants in the United States. Another major issue with coal burning is sulfur dioxide (SO2). In the atmosphere, sulfur dioxide becomes sulfuric acid. That, in turn, becomes a contributor to acid rain and smog. Other coal issues include mercury pollution, underground mind drainage, and major surface disruptions caused by open-pit mines.
 Despite this, coal is the best source of energy. It is the cheapest per unit of energy. It is also quite an industry. The US is one of the major producers of coal worldwide. There are over 6000 coal mines in the US alone. The former Soviet Union is also a major producer of coal. And coal reserves are large enough that, at the rate it is being mined, production should be able to continue for a few hundred years.
Despite this, coal is the best source of energy. It is the cheapest per unit of energy. It is also quite an industry. The US is one of the major producers of coal worldwide. There are over 6000 coal mines in the US alone. The former Soviet Union is also a major producer of coal. And coal reserves are large enough that, at the rate it is being mined, production should be able to continue for a few hundred years.So, the problem is, do we continue using coal? Do we continue burning coal, producing greenhouse gases and acid rain? Or do we look for alternate sources of energy, which is inevitable anyway?
Tuesday, September 25, 2007
Have gas? We do!
This is the second of the series of energy related blogs. In this post I will discuss natural gas. My main reference for this post is Kenneth S. Deffeyes' Beyond Oil.
A few things should be noted about natural gas:
1. It is produced from the decay of organic material in the absence of air.
2. It is mainly methane, but contains quantities of ethane, butane, propane, nitrogen, and helium.
3. It can be mined for a profit from rock that isn't easily permeable by oil.
Natural gas comes from two sources, "conventional" and "unconventional." Conventional gas comes from standard sources, generally mining of some form or another. Unconventional gas comes from other sources, such as swamp gas or coal beds.
Conventional gas production comes in three varieties. Solution gas is dissolved in oil and comes out of solution when the pressure drops. Basically, when the oil pocket is breached, the gas held in solution separates. A useful analogy would be carbonated beverages. Mining the oil in such pockets is not terribly profitable, but the games comes free. Production of natural gas from these types of mines previously kept gas prices down.
Gas caps form above oil reservoirs when there is more gas than the oil can dissolve. Miners can choose to get the oil first, leaving the gas for later production. Mining from such pockets is more effective at recovering larger percentages of oil, as well. As opposed to the 20-40% from solution gas, mining from gas caps recovers up to 50% of the oil contained therein.
The final conventional production is gas found below the depth where oil is located. Oil is found between 7500-15000 feet. Below that, oil deposits are quite rare. These pockets of gas are very good money makers for miners seeking the fossil fuel.
Unconventional gas production comes from four sources: swamp gas, coal bed gas, basin-center gas, and fractured shales. Swamp gas, as it is called, it produced by a bacteria which converts organic matter into methane gas. This would be the same bacteria which resides in the digestive tract. Some pockets of swamp gas can be commercially viable, but have no attracted the attention of major companies. However, since it is relatively easy to access, such deposits are quite rewarding for smaller countries.
Coal bed gas comes from the coal itself. As the coal is natural material, the decomposition of it produces the natural gas. However, the only reason such deposits exist is the sheer size of the plant matter used in the production of coal. Despite this, coal beds have managed to produce nearly 8% of the United States natural gas production.
Basin center gas is that which has completely permeated the host rock. However, this is not as viable a source of gas as, say, the coal beds. The natural gas is much harder to get and there never seems to be enough to completely saturate less-porous host rocks. By the same token, fractured shale gas can be equally as difficult to come by. More porous rocks like sandstone can become more saturated. However, the fractures in the rock produce wells that can be tapped slowly.
The use of natural gas is nothing new. Homes have stoves that use natural gas. Grills burn propane, a form of liquefied natural gas. Natural gas is also the third largest product used for the generation of electricity, after coal and nuclear power. Overall, it generates just over 600 kilowatt-hours of energy annually in the United States. But natural gas is also used for heating and industrial use. A portion of it also goes to make fertilizers because of the hydrogen that can be produced from natural gas. Since it has come to be used, in large part, for the production of electricity, the reserves of natural gas have been severely depleted.
Another interesting use for natural gas is in the automobile industry. The technology exists to use natural gas as fuel, instead of oil. Overall, these cars would use less than the equivalent of ten gallons of oil. A couple other benefits exist, such as the production of less carbon-dioxide and a high octane rating. (This basically means that it much reach a relatively high temperature before it spontaneously ignites.) However, the gas must be contained under high pressure. That also leads to another issue, that of refueling. The number of refueling stations for natural gas-run vehicles is scant, to say the least. They require specialized equipment, because of the high pressures. But this, in turn, exacerbates the problem. Deffeyes says, "individuals will not buy natural gas-powered vehicles because there are no filling stations; filling stations don't exist because nobody own natural gas powered vehicles" (Deffeyes 58).
As you can see, natural gas has its uses. It heats our homes and office buildings. It produces electricity for our gadgets. It can even be used to run our cars. However, reserves are dropping worldwide. Eventually, we will run out of easily accessable reserves and will need to find other methods to produce natural gas. Whether we will turn to the "unconventional" methods is yet to be known.
Do you have any thoughts on the matter? Is there an alternative for natural gas? Should we be reducing our reliance on this particular fossil fuel? Or should we simply continue as we have been?
A few things should be noted about natural gas:
1. It is produced from the decay of organic material in the absence of air.
2. It is mainly methane, but contains quantities of ethane, butane, propane, nitrogen, and helium.
3. It can be mined for a profit from rock that isn't easily permeable by oil.
Natural gas comes from two sources, "conventional" and "unconventional." Conventional gas comes from standard sources, generally mining of some form or another. Unconventional gas comes from other sources, such as swamp gas or coal beds.
Conventional gas production comes in three varieties. Solution gas is dissolved in oil and comes out of solution when the pressure drops. Basically, when the oil pocket is breached, the gas held in solution separates. A useful analogy would be carbonated beverages. Mining the oil in such pockets is not terribly profitable, but the games comes free. Production of natural gas from these types of mines previously kept gas prices down.
Gas caps form above oil reservoirs when there is more gas than the oil can dissolve. Miners can choose to get the oil first, leaving the gas for later production. Mining from such pockets is more effective at recovering larger percentages of oil, as well. As opposed to the 20-40% from solution gas, mining from gas caps recovers up to 50% of the oil contained therein.
The final conventional production is gas found below the depth where oil is located. Oil is found between 7500-15000 feet. Below that, oil deposits are quite rare. These pockets of gas are very good money makers for miners seeking the fossil fuel.
Unconventional gas production comes from four sources: swamp gas, coal bed gas, basin-center gas, and fractured shales. Swamp gas, as it is called, it produced by a bacteria which converts organic matter into methane gas. This would be the same bacteria which resides in the digestive tract. Some pockets of swamp gas can be commercially viable, but have no attracted the attention of major companies. However, since it is relatively easy to access, such deposits are quite rewarding for smaller countries.
Coal bed gas comes from the coal itself. As the coal is natural material, the decomposition of it produces the natural gas. However, the only reason such deposits exist is the sheer size of the plant matter used in the production of coal. Despite this, coal beds have managed to produce nearly 8% of the United States natural gas production.
Basin center gas is that which has completely permeated the host rock. However, this is not as viable a source of gas as, say, the coal beds. The natural gas is much harder to get and there never seems to be enough to completely saturate less-porous host rocks. By the same token, fractured shale gas can be equally as difficult to come by. More porous rocks like sandstone can become more saturated. However, the fractures in the rock produce wells that can be tapped slowly.
The use of natural gas is nothing new. Homes have stoves that use natural gas. Grills burn propane, a form of liquefied natural gas. Natural gas is also the third largest product used for the generation of electricity, after coal and nuclear power. Overall, it generates just over 600 kilowatt-hours of energy annually in the United States. But natural gas is also used for heating and industrial use. A portion of it also goes to make fertilizers because of the hydrogen that can be produced from natural gas. Since it has come to be used, in large part, for the production of electricity, the reserves of natural gas have been severely depleted.
Another interesting use for natural gas is in the automobile industry. The technology exists to use natural gas as fuel, instead of oil. Overall, these cars would use less than the equivalent of ten gallons of oil. A couple other benefits exist, such as the production of less carbon-dioxide and a high octane rating. (This basically means that it much reach a relatively high temperature before it spontaneously ignites.) However, the gas must be contained under high pressure. That also leads to another issue, that of refueling. The number of refueling stations for natural gas-run vehicles is scant, to say the least. They require specialized equipment, because of the high pressures. But this, in turn, exacerbates the problem. Deffeyes says, "individuals will not buy natural gas-powered vehicles because there are no filling stations; filling stations don't exist because nobody own natural gas powered vehicles" (Deffeyes 58).
As you can see, natural gas has its uses. It heats our homes and office buildings. It produces electricity for our gadgets. It can even be used to run our cars. However, reserves are dropping worldwide. Eventually, we will run out of easily accessable reserves and will need to find other methods to produce natural gas. Whether we will turn to the "unconventional" methods is yet to be known.
Do you have any thoughts on the matter? Is there an alternative for natural gas? Should we be reducing our reliance on this particular fossil fuel? Or should we simply continue as we have been?
Thursday, September 20, 2007
The Explosive Past and Productive Present - Nuclear Power
I have decided to do a small series or posts about energy. This includes energy sources as well as energy issues. This first post will cover nuclear power. I intend to discuss the benefits of nuclear energy, as well as one or two reactor types. For that, I will contrast the common pressurized water reactor to the "reactor cooled by water and moderated by graphite" (RBMK) reactor used for the Chernobyl reactor.
Chernobyl. The name sends shivers down the spines of any who know about what happened there. Chernobyl. A nuclear power station in the Ukraine whose #4 reactor exploded, releasing nuclear fallout over a wide swath of land. It is over 20 years since the explosion and there are still issues with radioactivity. This catastrophe is, perhaps, the paradigm of what can go wrong with nuclear reactors. It might even be enough to turn someone off the idea of nuclear power completely. However, as horrific as the Chernobyl incident was, the reactor used was poorly designed.
All nuclear reactors use particles, such as neutrons, to bombard fuel cells, such as Uranium. The effect of this process is the splitting of the target atom, releasing energy and neutrons. It is a chain reaction. Some of the energy released is radiation, in the form of heat. In order to ensure the continued production of energy, all reactors use a sort of buffer, which slows down the neutron sufficiently that it has a greater chance of hitting the target atom(s). In most American and Canadian reactors, this is water. The RBMK reactor used carbon in the form of graphite. (Reactor Design)
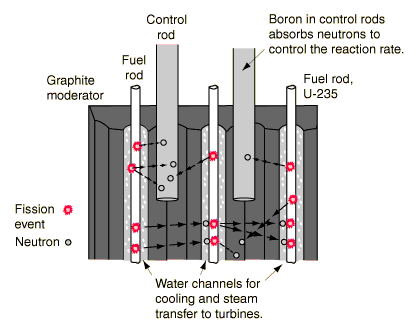 The design technology of the RBMK reactor was nearly 45 years outdated. It was, in fact, the same technology used by Enrico Fermi in 1942. As such, there were two major design flaws. The first was the carbon used as a moderator.
The design technology of the RBMK reactor was nearly 45 years outdated. It was, in fact, the same technology used by Enrico Fermi in 1942. As such, there were two major design flaws. The first was the carbon used as a moderator.
It does not stand up well under high pressures. Not only that, it burns in the core when exposed to air. The burning increases the speed of the neutrons, creating even more heat. To prevent this from happened, air had to be kept out of the reactor core. The second major problem with the RBMK design was its decided lack of containment. There
was a seal designed to keep pressure in, but nothing to protect and shield in the case of an explosion.
 Modern nuclear reactors, such as those used in America and Canada, are much safer than the RBMK design. Both designs are pressurized water reactors (PWRs). Instead of graphite, they use water to moderate the reaction speed and efficiency. They work via a double coolant system. The water in the primary coolant system, around the core, is pressurized so that it can remain liquid above its natural boiling point. In US reactors, ordinary water is used. On the other hand, Canadian reactors use heavy water (water that contains deuterium), which is a slightly more effective moderator.
Modern nuclear reactors, such as those used in America and Canada, are much safer than the RBMK design. Both designs are pressurized water reactors (PWRs). Instead of graphite, they use water to moderate the reaction speed and efficiency. They work via a double coolant system. The water in the primary coolant system, around the core, is pressurized so that it can remain liquid above its natural boiling point. In US reactors, ordinary water is used. On the other hand, Canadian reactors use heavy water (water that contains deuterium), which is a slightly more effective moderator.
The secondary coolant system uses the heat from the primary system to turn the water flowing through the pipes into steam. That steam is then used to rotate a turbine, producing electricity in a generator. The water is then run over a condenser, returning it to a liquid state so that the process may be repeated. The only water in the system which is irradiated is that in the primary coolant system. Water used in the secondary system does not come in any direct contact with the radioactive water. Additionally, each reactor is surrounded by a steel-reinforced concrete container. This is a safety measure designed to contain the escape of radiation in the event of an accident. It is the last of a line of defenses meant to protect and reduce radioactive fallout.
Of course, it goes without saying that even the safest reactor has the possibility of malfunctioning. So, then, the question is, why even consider nuclear power? What are the benefits of it, if there are any? Well, for one, they are more environmentally friendly that coal burning power plants. Additionally, the 103 nuclear plants have consistently produced about 20% of our power output for the past decade. According to a National Geographic article, the problem with cheaper coal plants "is the two billion tons of climate-warming carbon dioxide spewing skyward every year... The Nuclear Energy Institute estimates that without nuclear power playing its current role in the generation of electricity, the U.S. would spew 29 percent—190 million metric tons—more carbon than it does now."
Aside from being more environmentally friendly, reactor technology is always advancing. In a few years, designs could lead to reactors that work more efficiently at higher temperatures. Such "intense nuclear reactions at such temperatures would leave waste that, compared to today's, is less toxic and lasts for a shorter period of time." In the long run, this means storage of these materials could be handled more easily. Not only that, the fuel would not be nearly as useful to terrorist groups looking to steal it.
Other countries use nuclear power. France, for example, derives nearly 80% of her electricity from nuclear power plants. Despite China's race to build coal plants, it also has designs for several nuclear plants. India, a nation with a massive population, has a significant number of reactors, with several more that are being built. In the US, a poll found that 59% of the population is in favor of nuclear power. But what about other, less dangerous, less expensive, forms of power?
~Interminable Immediacy
Chernobyl. The name sends shivers down the spines of any who know about what happened there. Chernobyl. A nuclear power station in the Ukraine whose #4 reactor exploded, releasing nuclear fallout over a wide swath of land. It is over 20 years since the explosion and there are still issues with radioactivity. This catastrophe is, perhaps, the paradigm of what can go wrong with nuclear reactors. It might even be enough to turn someone off the idea of nuclear power completely. However, as horrific as the Chernobyl incident was, the reactor used was poorly designed.
All nuclear reactors use particles, such as neutrons, to bombard fuel cells, such as Uranium. The effect of this process is the splitting of the target atom, releasing energy and neutrons. It is a chain reaction. Some of the energy released is radiation, in the form of heat. In order to ensure the continued production of energy, all reactors use a sort of buffer, which slows down the neutron sufficiently that it has a greater chance of hitting the target atom(s). In most American and Canadian reactors, this is water. The RBMK reactor used carbon in the form of graphite. (Reactor Design)
 The design technology of the RBMK reactor was nearly 45 years outdated. It was, in fact, the same technology used by Enrico Fermi in 1942. As such, there were two major design flaws. The first was the carbon used as a moderator.
The design technology of the RBMK reactor was nearly 45 years outdated. It was, in fact, the same technology used by Enrico Fermi in 1942. As such, there were two major design flaws. The first was the carbon used as a moderator.It does not stand up well under high pressures. Not only that, it burns in the core when exposed to air. The burning increases the speed of the neutrons, creating even more heat. To prevent this from happened, air had to be kept out of the reactor core. The second major problem with the RBMK design was its decided lack of containment. There
was a seal designed to keep pressure in, but nothing to protect and shield in the case of an explosion.
 Modern nuclear reactors, such as those used in America and Canada, are much safer than the RBMK design. Both designs are pressurized water reactors (PWRs). Instead of graphite, they use water to moderate the reaction speed and efficiency. They work via a double coolant system. The water in the primary coolant system, around the core, is pressurized so that it can remain liquid above its natural boiling point. In US reactors, ordinary water is used. On the other hand, Canadian reactors use heavy water (water that contains deuterium), which is a slightly more effective moderator.
Modern nuclear reactors, such as those used in America and Canada, are much safer than the RBMK design. Both designs are pressurized water reactors (PWRs). Instead of graphite, they use water to moderate the reaction speed and efficiency. They work via a double coolant system. The water in the primary coolant system, around the core, is pressurized so that it can remain liquid above its natural boiling point. In US reactors, ordinary water is used. On the other hand, Canadian reactors use heavy water (water that contains deuterium), which is a slightly more effective moderator.The secondary coolant system uses the heat from the primary system to turn the water flowing through the pipes into steam. That steam is then used to rotate a turbine, producing electricity in a generator. The water is then run over a condenser, returning it to a liquid state so that the process may be repeated. The only water in the system which is irradiated is that in the primary coolant system. Water used in the secondary system does not come in any direct contact with the radioactive water. Additionally, each reactor is surrounded by a steel-reinforced concrete container. This is a safety measure designed to contain the escape of radiation in the event of an accident. It is the last of a line of defenses meant to protect and reduce radioactive fallout.
Of course, it goes without saying that even the safest reactor has the possibility of malfunctioning. So, then, the question is, why even consider nuclear power? What are the benefits of it, if there are any? Well, for one, they are more environmentally friendly that coal burning power plants. Additionally, the 103 nuclear plants have consistently produced about 20% of our power output for the past decade. According to a National Geographic article, the problem with cheaper coal plants "is the two billion tons of climate-warming carbon dioxide spewing skyward every year... The Nuclear Energy Institute estimates that without nuclear power playing its current role in the generation of electricity, the U.S. would spew 29 percent—190 million metric tons—more carbon than it does now."
Aside from being more environmentally friendly, reactor technology is always advancing. In a few years, designs could lead to reactors that work more efficiently at higher temperatures. Such "intense nuclear reactions at such temperatures would leave waste that, compared to today's, is less toxic and lasts for a shorter period of time." In the long run, this means storage of these materials could be handled more easily. Not only that, the fuel would not be nearly as useful to terrorist groups looking to steal it.
Other countries use nuclear power. France, for example, derives nearly 80% of her electricity from nuclear power plants. Despite China's race to build coal plants, it also has designs for several nuclear plants. India, a nation with a massive population, has a significant number of reactors, with several more that are being built. In the US, a poll found that 59% of the population is in favor of nuclear power. But what about other, less dangerous, less expensive, forms of power?
~Interminable Immediacy
Tuesday, September 18, 2007
The Science of Belief
Okay, so maybe today won't be so driven by facts and statistics. Today's post will be a musing on religion. With perhaps a few stats plugged in where relevant. I'm not entirely sure which way this post is going to go. It might just turn out like a regular blog post. I shall do my best to prevent that from happening, but I guarantee nothing.

Please note, the above chart is based solely on a sociological data.
Religion is a major part of modern society. From it we derive our value system, ethics, a model of how to live, and the hope of something more. Values? Ethics? We know what those are, right? Values help us determine what is right and wrong, while ethics help us determine how to live by our vales. Religious leaders provide examples of how we should live our lives as good people and good citizens. Finally, and perhaps most importantly, religion generally provides the hope of an afterlife, a promise that death is not an end.
But in some ways, religion is a social convention. It was "invented" by humans as a way to control the population, keep them in check, and, in some societies, to justify the amount of power held by certain individuals. By now, every rabid believer is probably slavering to get at me right now. How dare I call religion an invention! I do and I can. And at the same time, I can still have a strong faith. I have some points to make on the matter.
First of all, Hinduism is a religion based on castes. There are four of them: Brahmin, Kshatriya, Vaishya, and Sudra. The Brahmin and Kshatriya, priests and leaders respectively, were the highest classes in Hindu society. But there are two things here that I must point out. One of the Hindu creation myths states that the Brahmin came from the mouth of Purusha, the first man. That automatically sets them up as the leaders of society, giving them the most power. Whether this was so justify the Aryan power in India or simply a religious convention, it matters not. The religion puts a certain group above others in society, a direct contrast to a religion like Buddhism, which has no caste system.
Okay, Hinduism is just one religion. That doesn't mean all the others are like that. Well, allow me to reference an old religion, that of the Celts. Collectively, they worshiped nature deities and they had a priestly class. That class held the power in Celtic society, talking to the spirits and leading the people. What did the invading Romans do? They killed off all the priests, in order to break their hold. How about another example; the Egyptians. They believed their rulers were not just descended of the gods, but living gods in their own right. That belief gave them the right, the divine right, to rule. What about something a little more modern? For centuries, European rulers believed that God granted them the right to the monarchy.
 So, then, with divinely backed rulers in place, what now? Well, the Mesopotamians had an interesting relationship with the gods in their pantheon. They believed that if an individual messed up, the entire society would be punished for that person's mistake. Their kings were depicted being given the law by the chief god, Shamash (Hammurabi being the prime example in this case). Here, religion is used to make the people fearful enough to follow the law. Because if they didn't, everyone would be punished.
So, then, with divinely backed rulers in place, what now? Well, the Mesopotamians had an interesting relationship with the gods in their pantheon. They believed that if an individual messed up, the entire society would be punished for that person's mistake. Their kings were depicted being given the law by the chief god, Shamash (Hammurabi being the prime example in this case). Here, religion is used to make the people fearful enough to follow the law. Because if they didn't, everyone would be punished.
To quit referencing ancient religions, the same applies today. Jews, Christians, and Muslims have laws they must follow. For Jews, not only do the Ten Commandments apply, but the laws in Leviticus as well. For Christians, there are the Ten Commandments and directions from the popes and other past leaders. For Muslims, they have the Qu'ran and the precepts of Muhammad and the other caliphs.
You may not agree with me or you may. I have presented what evidence I could. Just remember, I am not bashing religion. It is a good thing in that can help people lead better lives and gives them hope. Hindus and Buddhists believe they will eventually escape the cycle of samsara. Jews, Christians, and Muslims believe in a heaven, where they will see God and meet the saints and holy people. Zoroastrians believe they will cross a bridge at death and if they are true, they will reach a good afterlife. People see these promises and strive for them.
Do you have any thoughts on the matter? Please feel free to discuss what I have written.

Please note, the above chart is based solely on a sociological data.
Religion is a major part of modern society. From it we derive our value system, ethics, a model of how to live, and the hope of something more. Values? Ethics? We know what those are, right? Values help us determine what is right and wrong, while ethics help us determine how to live by our vales. Religious leaders provide examples of how we should live our lives as good people and good citizens. Finally, and perhaps most importantly, religion generally provides the hope of an afterlife, a promise that death is not an end.
But in some ways, religion is a social convention. It was "invented" by humans as a way to control the population, keep them in check, and, in some societies, to justify the amount of power held by certain individuals. By now, every rabid believer is probably slavering to get at me right now. How dare I call religion an invention! I do and I can. And at the same time, I can still have a strong faith. I have some points to make on the matter.
First of all, Hinduism is a religion based on castes. There are four of them: Brahmin, Kshatriya, Vaishya, and Sudra. The Brahmin and Kshatriya, priests and leaders respectively, were the highest classes in Hindu society. But there are two things here that I must point out. One of the Hindu creation myths states that the Brahmin came from the mouth of Purusha, the first man. That automatically sets them up as the leaders of society, giving them the most power. Whether this was so justify the Aryan power in India or simply a religious convention, it matters not. The religion puts a certain group above others in society, a direct contrast to a religion like Buddhism, which has no caste system.
Okay, Hinduism is just one religion. That doesn't mean all the others are like that. Well, allow me to reference an old religion, that of the Celts. Collectively, they worshiped nature deities and they had a priestly class. That class held the power in Celtic society, talking to the spirits and leading the people. What did the invading Romans do? They killed off all the priests, in order to break their hold. How about another example; the Egyptians. They believed their rulers were not just descended of the gods, but living gods in their own right. That belief gave them the right, the divine right, to rule. What about something a little more modern? For centuries, European rulers believed that God granted them the right to the monarchy.
 So, then, with divinely backed rulers in place, what now? Well, the Mesopotamians had an interesting relationship with the gods in their pantheon. They believed that if an individual messed up, the entire society would be punished for that person's mistake. Their kings were depicted being given the law by the chief god, Shamash (Hammurabi being the prime example in this case). Here, religion is used to make the people fearful enough to follow the law. Because if they didn't, everyone would be punished.
So, then, with divinely backed rulers in place, what now? Well, the Mesopotamians had an interesting relationship with the gods in their pantheon. They believed that if an individual messed up, the entire society would be punished for that person's mistake. Their kings were depicted being given the law by the chief god, Shamash (Hammurabi being the prime example in this case). Here, religion is used to make the people fearful enough to follow the law. Because if they didn't, everyone would be punished.To quit referencing ancient religions, the same applies today. Jews, Christians, and Muslims have laws they must follow. For Jews, not only do the Ten Commandments apply, but the laws in Leviticus as well. For Christians, there are the Ten Commandments and directions from the popes and other past leaders. For Muslims, they have the Qu'ran and the precepts of Muhammad and the other caliphs.
You may not agree with me or you may. I have presented what evidence I could. Just remember, I am not bashing religion. It is a good thing in that can help people lead better lives and gives them hope. Hindus and Buddhists believe they will eventually escape the cycle of samsara. Jews, Christians, and Muslims believe in a heaven, where they will see God and meet the saints and holy people. Zoroastrians believe they will cross a bridge at death and if they are true, they will reach a good afterlife. People see these promises and strive for them.
Do you have any thoughts on the matter? Please feel free to discuss what I have written.
Thursday, September 13, 2007
I'd like "Things that go BANG!" for 1000.
Well, ladies and gentlemen, I am returning to topics that require at least some research. Hopefully, however, you will find this post to not be as dry as some of the others could be. At least for me, this one is quite interesting. So, should I kill the suspense and just tell you aready? Oh, all right. As you may have guessed, based on the title, this blog will be about explosive material.
First I will list some generic characteristics of explosives. Then I'll discuss the differences between the basic classes into which these materials are categorized. Finally, I might discuss some materials that fall into each category.
Explosive material is something that is chemically or energetically unstable. This means, under the appropriate conditions, these materials can produce heat and pressure changes. They are usually accompanied by a flash or loud noise. Explosives generally either undergo deflagration (decomposition due to rapid burning) or detonation (decomposition propagated by a concussive shockwave). Generally, they have less potential energy than petroleum fuels, but the pressure is created by the rapid energy release. Since the force released by explosives moves perpendicular to the explosion itself, shaped charges can be made to direct the blast.
The overall properties of any given explosive determine the class into which it is categorized. There are two main categories, high explosives and low explosives. Low explosives are materials such as black powder, smokeless powder, and flash powder. For the most part, these materials are use as propellants. Under normal circumstances, they deflagrate rather than detonate. However, if set off in a confined space, the effect produced can be similar to a detonation.
High explosives are materials such as nitroglycerin, TNT, and RDX. They are used in mining, demolition, and military warheads. This class of material will always detonate rather than deflagrate. This class of explosives can be further subdivided into primary and secondary explosives. Primary explosives can be detonated by shock, heat, or friction. Secondary explosives are generally resistant to such forces and are used to add power to blasting caps (devices used to detonate more powerful explosives.)

Black powder, or gun powder, is composed of a nitrate, charcoal, and sulfur. Each ingredient provides vital element to the mixture. The nitrate provides oxygen, the charcoal fuel, and the sulfur fuel and a lower ignition temperature. A typical use of black powder is firearm ammunition and pyrotechnics (fireworks).
Perhaps the most well-known explosive is nitroglycerin. It was first produced by Ascanio Sobrero in 1846. Nitroglycerin is a clear, slightly oily, highly unstable liquid. Of the more interesting uses for this material is as a medical vasodilator. In other words, it widens the blood vessels to reduce blood pressure. When taken, it can decrease blood pressure and increase heart rate. More commonly, however, it is absorbed into wood or a powedered material. This acts to stabilize the nitroglycerin. The product is then called Dynamite. In such a form it can be used for mining or demolition.
One last comment. Explosives are not something to be taken lightly, even the ones in the low explosive category. All of them should be handled with caution and properly contained.
~Interminable Immediacy
(sources:
Wikipedia Article
Fire and Safety)
First I will list some generic characteristics of explosives. Then I'll discuss the differences between the basic classes into which these materials are categorized. Finally, I might discuss some materials that fall into each category.
Explosive material is something that is chemically or energetically unstable. This means, under the appropriate conditions, these materials can produce heat and pressure changes. They are usually accompanied by a flash or loud noise. Explosives generally either undergo deflagration (decomposition due to rapid burning) or detonation (decomposition propagated by a concussive shockwave). Generally, they have less potential energy than petroleum fuels, but the pressure is created by the rapid energy release. Since the force released by explosives moves perpendicular to the explosion itself, shaped charges can be made to direct the blast.
The overall properties of any given explosive determine the class into which it is categorized. There are two main categories, high explosives and low explosives. Low explosives are materials such as black powder, smokeless powder, and flash powder. For the most part, these materials are use as propellants. Under normal circumstances, they deflagrate rather than detonate. However, if set off in a confined space, the effect produced can be similar to a detonation.
High explosives are materials such as nitroglycerin, TNT, and RDX. They are used in mining, demolition, and military warheads. This class of material will always detonate rather than deflagrate. This class of explosives can be further subdivided into primary and secondary explosives. Primary explosives can be detonated by shock, heat, or friction. Secondary explosives are generally resistant to such forces and are used to add power to blasting caps (devices used to detonate more powerful explosives.)

Black powder, or gun powder, is composed of a nitrate, charcoal, and sulfur. Each ingredient provides vital element to the mixture. The nitrate provides oxygen, the charcoal fuel, and the sulfur fuel and a lower ignition temperature. A typical use of black powder is firearm ammunition and pyrotechnics (fireworks).
Perhaps the most well-known explosive is nitroglycerin. It was first produced by Ascanio Sobrero in 1846. Nitroglycerin is a clear, slightly oily, highly unstable liquid. Of the more interesting uses for this material is as a medical vasodilator. In other words, it widens the blood vessels to reduce blood pressure. When taken, it can decrease blood pressure and increase heart rate. More commonly, however, it is absorbed into wood or a powedered material. This acts to stabilize the nitroglycerin. The product is then called Dynamite. In such a form it can be used for mining or demolition.
One last comment. Explosives are not something to be taken lightly, even the ones in the low explosive category. All of them should be handled with caution and properly contained.
~Interminable Immediacy
(sources:
Wikipedia Article
Fire and Safety)
Thursday, September 6, 2007
Do you D.A.R.E.?
Instead of my regularly scheduled discussion of a completely different issue, I have decided that today's entry will revolve around drugs and drug abuse. Obviously I refer mainly to illegal drugs, such as cocaine, marijuana, methamphetamines, and hallucinogens. However, there are some prescription and over-the-counter drugs that can be taken improperly. I will try to refrain from discussing those drugs in this blog. Also, please note, I am not taking a stand for either side of the argument. My personal preferences aside, this is simply to state facts.
Anyone who has taken a Drug Abuse Resistance Education (D.A.R.E.) class knows the dangers of drugs. But allow me to go over the basics one more time. Drugs are taken in various manners, from inhalation, to ingestion, to injection. When taken in sufficient quantities, they induce a state of euphoria known as a "high." In some cases, that high might also lead to a low. Eventually, however, the euphoria passes and the individual must somehow obtain more of the drug to repeat the effect. Over time, the dosage required to create the effect increases as the body becomes resistant to the drug. Most drugs also carry the risk of dependency.
Having re-iterated the basics of drug use, perhaps it is time to list a few interesting facts, courtesy of SADD. SADD was previously mentioned in the blog about alcohol.
The first drug I would like to discuss is cocaine. Cocaine is a drug that can be smoked, snorted, or injected. Initially, it was used by doctors because of its ability to act as an anesthetic and to limit bleeding.
Cannabis is a general term for any drug that comes from the cannabis sativa plant. These products include marijuana, hashish, and sinsemilla. They are all psychoactive, containing the chemical THC. Marijuana, the most common from of cannabis, is generally smoked. But it can also be brewed into a tea and ingested.
The last drug I am going to discuss is methamphetamine, or meth. Meth affects the central nervous system and can be administered by injection, ingestion, smoking, and snorting. It increases activity and decreases appetite. It has limited use in the medical field for narcolepsy, attention deficit disorders, and obesity.
Please feel free to add any other information you have or to discuss the use/effects of drugs.
~Interminable Immediacy
Anyone who has taken a Drug Abuse Resistance Education (D.A.R.E.) class knows the dangers of drugs. But allow me to go over the basics one more time. Drugs are taken in various manners, from inhalation, to ingestion, to injection. When taken in sufficient quantities, they induce a state of euphoria known as a "high." In some cases, that high might also lead to a low. Eventually, however, the euphoria passes and the individual must somehow obtain more of the drug to repeat the effect. Over time, the dosage required to create the effect increases as the body becomes resistant to the drug. Most drugs also carry the risk of dependency.
Having re-iterated the basics of drug use, perhaps it is time to list a few interesting facts, courtesy of SADD. SADD was previously mentioned in the blog about alcohol.
- Half of teens (50%) have tried an illicit drug by the time they finish high school.
- Two-fifths of 8th graders (41%) and almost three fourths of all 10th graders (73%) consider marijuana to be easily accessible; compare these figures with the percentage of 12th graders - 86%.
- Nationwide, 25.4% of students had been offered, sold, or given an illegal drug by someone on school property.
- Youths ages 12-17 who believed their parents would strongly disapprove of their using a particular substance were less likely to use that substance than were youths who believed their parents would somewhat disapprove or neither approve nor disapprove.
The first drug I would like to discuss is cocaine. Cocaine is a drug that can be smoked, snorted, or injected. Initially, it was used by doctors because of its ability to act as an anesthetic and to limit bleeding.
- Approximately 8.8% of college students and 14.3% of young adults (ages 19-28) surveyed in 2005 reported lifetime use of cocaine.
- The 2005 National Survey on Drug Use and Health (NSDUH) indicated that there were 872,000 persons aged 12 or older who had used cocaine for the first time within the past 12 months. This is a statistically significant reduction from 2002 when there were more than one million past year cocaine initiates.
Cannabis is a general term for any drug that comes from the cannabis sativa plant. These products include marijuana, hashish, and sinsemilla. They are all psychoactive, containing the chemical THC. Marijuana, the most common from of cannabis, is generally smoked. But it can also be brewed into a tea and ingested.
- The Youth Risk Behavior Surveillance (YRBS) study by the Centers for Disease Control (CDC) surveys high school students on several risk factors including drug and alcohol use. Results of the 2005 survey indicate that 38.4% of high school students reported using marijuana at some point in their lifetimes. Additional YRBS results indicate that 20.2% of students surveyed in 2005 reported current (past month) use of marijuana.
- According the to Bureau of Justice Statistics, approximately 77.6% of State prisoners and 71.2% of Federal prisoners surveyed in 2004 that they used marijuana/hashish at some point in their life.
The last drug I am going to discuss is methamphetamine, or meth. Meth affects the central nervous system and can be administered by injection, ingestion, smoking, and snorting. It increases activity and decreases appetite. It has limited use in the medical field for narcolepsy, attention deficit disorders, and obesity.
- Approximately 4.1% of college students and 8.3% of young adultes (ages 19-28) surveyed in 2005 reported lifetime use of amphetamines.
Please feel free to add any other information you have or to discuss the use/effects of drugs.
~Interminable Immediacy
Wednesday, September 5, 2007
Phobias - Are you afraid?
Overall, approximately 18.1% of the United States population suffers from some sort of anxiety disorder. The people who suffer from such disorders are 3-5 times more likely to visit the doctor, 6 times more likely to be hospitalized for psychiatric disorders. (http://www.adaa.org/) Aside from social disorders, or obsessive-compulsive disorders, a portion of the population lives with constant, unreasoning fear. These are known as phobias. According to the Anxiety Disorders Association of America (ADAA), a phobia is characterized by “strong, irrational, involuntary fear reactions to a particular object, place, or situation…”
My main focus is these phobias. On average, about 10% of the American population suffer from a phobia of some kind. What is interesting is that women are more susceptible to phobic disorders than men. The problem with having a phobia is that the fear makes no sense in the context of the every day. But there is no way for the phobic to control or stop the reactions he or she has.
Phobias can disrupt the everyday life of an individual, lessen his or her efficiency, lower self-esteem, and strain relationships. More often than not, the onset of a phobia is sudden. It can occur at any point in a person’s life, but usually develop during childhood or adolescence. They can spring up due to a traumatic or stressful situation. But they can also occur during situations that were previously of a benign nature to the individual. According to James S. Nairne, in Psychology Fourth Edition, phobias derive from four categories: 1) animals, 2) natural environments, 3) blood/injection/injury, and 4) specific situations (Nairne 482). There are, of course, phobias that do not fit into any of the four categories, such as fear of baseballs, clowns, choking, or costumed characters.
The first type, Zoophobias (animal fears), is quite self-explanatory. Arachnophobia, the fear of arachnids such as spiders and scorpions is one common example from this category. Another example would be Ophidiophobia, or the fear of snakes. Both fears have a certain basis in truth, as some arachnids and some snakes are poisonous. However, phobics don’t fear just the dangerous creatures, they fear all of them.
Natural environment phobias, the second group, cover fears such as Acrophobia, the fear of heights, and Astraphobia, the fear of thunder and lightening. Astraphobia is most common among children, though teens and adults can exhibit symptoms of it as well. Often, dogs and cats also show signs of being astraphobic. Most of these fears develop from personal experience or behavioral conditioning.
Trypanophobia, the fear of medical procedures involving injections or hypodermic needles, is an example of the third category of phobia. These fears, blood, injection, and injury, can be brought on be reflex. In other words, they see a close family member show fear at these things. There are other phobias that fall into this category, as well. Some of them refer specifically to an object, such as the fear of needles or pain, like Algophobia. Others have a decidedly medical aspect to them.
Finally, phobias such as Claustrophobia and Nyctophobia, fall into the situational category. Claustrophobia is the fear of small, confined spaces. It can be anything from an elevator to an airplane, to a train. Sometimes it develops because of a panic attack while in a confined space, but that is not always so. Children commonly exhibit nyctophobia, the fear of the dark. In most instances, the fear recedes as the individual ages.
There are other fears as well, that do not easily fall into the aforementioned categories. Notable ones among those are Triskaidekaphobia, fear of the number 13, and Coulrophobia, fear of clowns. Thankfully, there are ways to treat phobias. Some of them include exposure to the cause of their phobia, therapy, and medication. The type of treatment depends on the individual, as to the results.
To conclude, phobias can be debilitating. They are unreasoning fears that make little to no sense in every day life. They can occur suddenly, but they can also be treated. Do you have a phobia? Or do you know someone with a phobia? What sort of effects to their fears have on their lives? Please feel free to discuss this.
My main focus is these phobias. On average, about 10% of the American population suffer from a phobia of some kind. What is interesting is that women are more susceptible to phobic disorders than men. The problem with having a phobia is that the fear makes no sense in the context of the every day. But there is no way for the phobic to control or stop the reactions he or she has.
Phobias can disrupt the everyday life of an individual, lessen his or her efficiency, lower self-esteem, and strain relationships. More often than not, the onset of a phobia is sudden. It can occur at any point in a person’s life, but usually develop during childhood or adolescence. They can spring up due to a traumatic or stressful situation. But they can also occur during situations that were previously of a benign nature to the individual. According to James S. Nairne, in Psychology Fourth Edition, phobias derive from four categories: 1) animals, 2) natural environments, 3) blood/injection/injury, and 4) specific situations (Nairne 482). There are, of course, phobias that do not fit into any of the four categories, such as fear of baseballs, clowns, choking, or costumed characters.
The first type, Zoophobias (animal fears), is quite self-explanatory. Arachnophobia, the fear of arachnids such as spiders and scorpions is one common example from this category. Another example would be Ophidiophobia, or the fear of snakes. Both fears have a certain basis in truth, as some arachnids and some snakes are poisonous. However, phobics don’t fear just the dangerous creatures, they fear all of them.
Natural environment phobias, the second group, cover fears such as Acrophobia, the fear of heights, and Astraphobia, the fear of thunder and lightening. Astraphobia is most common among children, though teens and adults can exhibit symptoms of it as well. Often, dogs and cats also show signs of being astraphobic. Most of these fears develop from personal experience or behavioral conditioning.
Trypanophobia, the fear of medical procedures involving injections or hypodermic needles, is an example of the third category of phobia. These fears, blood, injection, and injury, can be brought on be reflex. In other words, they see a close family member show fear at these things. There are other phobias that fall into this category, as well. Some of them refer specifically to an object, such as the fear of needles or pain, like Algophobia. Others have a decidedly medical aspect to them.
Finally, phobias such as Claustrophobia and Nyctophobia, fall into the situational category. Claustrophobia is the fear of small, confined spaces. It can be anything from an elevator to an airplane, to a train. Sometimes it develops because of a panic attack while in a confined space, but that is not always so. Children commonly exhibit nyctophobia, the fear of the dark. In most instances, the fear recedes as the individual ages.
There are other fears as well, that do not easily fall into the aforementioned categories. Notable ones among those are Triskaidekaphobia, fear of the number 13, and Coulrophobia, fear of clowns. Thankfully, there are ways to treat phobias. Some of them include exposure to the cause of their phobia, therapy, and medication. The type of treatment depends on the individual, as to the results.
To conclude, phobias can be debilitating. They are unreasoning fears that make little to no sense in every day life. They can occur suddenly, but they can also be treated. Do you have a phobia? Or do you know someone with a phobia? What sort of effects to their fears have on their lives? Please feel free to discuss this.
Wednesday, August 29, 2007
Drinking Responsibly
I have decided, for this post, that I shall discuss something that I think is vitally important in this day and age. As it says in the title, it's drinking responsibly. This mainly concerns the young people in our nation, those between the ages of 18-22. I suppose there are a couple issues concerning this topic that I'd like to address. The first topic deals with the seemingly overwhelming obsession with drinking. Second on the list, but no less important, is the discussion of consequences. Last on the list is how parents can help prevent the abuse of alcohol by their children.
Here are a few statistics to get things started. These come from Students Against Destructive Decisions, or SADD. SADD originally developed as a student-run program to teach about the dangers of drinking and driving. Since then, they have taken other issues into account. Their mission statement is simply this: "To provide students with the best prevention and intervention tools possible to deal with the issues of underage drinking, other drug use, impaired driving, and other destructive decisions."
(http://www.sadd.org/stats.htm#underage)
Why is it considered "cool" to drink? Since I have never given into the pressure, I cannot speak from personal experience. However, I can guess. In a social situation, such as a party that has alcohol, there is peer pressure. Comments like, "Come on, everyone is doing it," can sway enough the most level-headed person. We give into our peers so that we can feel like we are accepted, like we fit in. And that makes us feel good, makes us feel cool. There is also the fact that some young adults see their parents drinking irresponsibly from a young age, so they don't know it is wrong. To them, it is normal. For others, there is a certain amount of curiosity and impatience concerning their ability to drink legally.
None of these are good reasons to drink irresponsibly. None of them are good reasons to drink at all, really. And not taking care in such situations can lead to dire consequences, some of which can be life threatening. The most common example is that of drunk driving. The impaired ability to function can lead to accidents with can severely injure, maim, or kill the individuals involved. We know this to be true. However, there are other consequences as well.
Alcohol can have adverse reactions with mixed with medications. There are over 150 medications that are not to be taken with alcohol. Something as common as acetaminophen (Tylenol) reacts with alcohol and can lead to serious liver problems. Drinking alcohol while using sleeping pills can negate the effects of the drug. These are just a very few of the consequences that mixing medication and alcohol can have.
Drinking can lead to birth defects. Children exposed to alcohol in the womb can have behavioral and learning disabilities. Fetal Alcohol Syndrome (FAS), the most common of alcohol-related birth defects, leads to severe physical, mental, and behavioral problems. The reason pregnant or potentially pregnant women are cautioned against the consumption of alcoholic beverages is because scientists do not know how much alcohol it takes to affect fetal development. Therefore, it is safer to refrain from any and all liquor if there is the remotest possibility of pregnancy.
Alcoholics can also have problems with their own bodies. Excessive drinking can lead to liver disease and failure. The liver is essential to the efficiency of the body, as it helps filter toxins from the blood stream. While moderate drinking can help reduce the potentiality of a heart attack, heavy drinking can cause an increase in the risk of heart disease, high blood pressure, and stroke. Heavy drinking can increase the risk for certain types of cancer, such as mouth, throat, breast, and colon cancer. Finally, long-term heavy drinking can lead to inflammation of the pancreas, called Pancreatitis. The pancreas produces insulin to help regulate blood sugar and it helps digest the food we eat. (www.collegedrinkingprevention.gov)
So, as we can see, the effects of irresponsible, heavy drinking can be quite severe. But what can be done to stop it? This is where parents come in, parents and peers. Parents can talk to their children about drinking. They can discuss the consequences and ways to avoid doing it in situations where their peers are drinking. Not only that, they can help build good drinking habits. For example, once the child reaches a certain age, say 14-15, the parent can invite their child to have a small glass of wine with dinner. From there, it may lead to some champagne on holidays. It can be taken further to include extremely small amounts of other drinks. However it develops, this introduction to alcohol will help teach the child that it is acceptable to drink in certain situations, in appropriate amounts.
But friends and peers can help as well. They can encourage their friends to think about their actions and to abstain from excessive, inappropriate, irresponsible drinking. Peers have more of a voice than they think they do. Especially when they are concerned for their friends. If they think their friend isn't acting responsibly, they can always go talk to a parent or councilor about it. They may have to deal with their friend(s) being angry, but better they are angry than continuing on a path to alcoholism.
To finalize, alcohol abuse is an ever present occurrence. It can lead to severe physical, social, and legal consequences. But steps can, and should, be taken to prevent an increase in such behavior.
~Interminable Immediacy
Here are a few statistics to get things started. These come from Students Against Destructive Decisions, or SADD. SADD originally developed as a student-run program to teach about the dangers of drinking and driving. Since then, they have taken other issues into account. Their mission statement is simply this: "To provide students with the best prevention and intervention tools possible to deal with the issues of underage drinking, other drug use, impaired driving, and other destructive decisions."
(http://www.sadd.org/stats.htm#underage)
- Young adults ages 18-22 enrolled full time in college were more likely than their peers not enrolled full time to use alcohol in the past month, to binge drink, and to drink heavily.
- Adults 21 or older who first used alcohol before age 21 were more likely than adults who had their first drink at 21 or older to be classified with alcohol dependence or abuse (9.6% vs 2.1%).
- In 2005, 85.6% of youths 12-17 reported that they strongly or somewhat disapprove of peers having one or two drinks of an alcoholic beverage nearly every day.
Why is it considered "cool" to drink? Since I have never given into the pressure, I cannot speak from personal experience. However, I can guess. In a social situation, such as a party that has alcohol, there is peer pressure. Comments like, "Come on, everyone is doing it," can sway enough the most level-headed person. We give into our peers so that we can feel like we are accepted, like we fit in. And that makes us feel good, makes us feel cool. There is also the fact that some young adults see their parents drinking irresponsibly from a young age, so they don't know it is wrong. To them, it is normal. For others, there is a certain amount of curiosity and impatience concerning their ability to drink legally.
None of these are good reasons to drink irresponsibly. None of them are good reasons to drink at all, really. And not taking care in such situations can lead to dire consequences, some of which can be life threatening. The most common example is that of drunk driving. The impaired ability to function can lead to accidents with can severely injure, maim, or kill the individuals involved. We know this to be true. However, there are other consequences as well.
Alcohol can have adverse reactions with mixed with medications. There are over 150 medications that are not to be taken with alcohol. Something as common as acetaminophen (Tylenol) reacts with alcohol and can lead to serious liver problems. Drinking alcohol while using sleeping pills can negate the effects of the drug. These are just a very few of the consequences that mixing medication and alcohol can have.
Drinking can lead to birth defects. Children exposed to alcohol in the womb can have behavioral and learning disabilities. Fetal Alcohol Syndrome (FAS), the most common of alcohol-related birth defects, leads to severe physical, mental, and behavioral problems. The reason pregnant or potentially pregnant women are cautioned against the consumption of alcoholic beverages is because scientists do not know how much alcohol it takes to affect fetal development. Therefore, it is safer to refrain from any and all liquor if there is the remotest possibility of pregnancy.
Alcoholics can also have problems with their own bodies. Excessive drinking can lead to liver disease and failure. The liver is essential to the efficiency of the body, as it helps filter toxins from the blood stream. While moderate drinking can help reduce the potentiality of a heart attack, heavy drinking can cause an increase in the risk of heart disease, high blood pressure, and stroke. Heavy drinking can increase the risk for certain types of cancer, such as mouth, throat, breast, and colon cancer. Finally, long-term heavy drinking can lead to inflammation of the pancreas, called Pancreatitis. The pancreas produces insulin to help regulate blood sugar and it helps digest the food we eat. (www.collegedrinkingprevention.gov)
So, as we can see, the effects of irresponsible, heavy drinking can be quite severe. But what can be done to stop it? This is where parents come in, parents and peers. Parents can talk to their children about drinking. They can discuss the consequences and ways to avoid doing it in situations where their peers are drinking. Not only that, they can help build good drinking habits. For example, once the child reaches a certain age, say 14-15, the parent can invite their child to have a small glass of wine with dinner. From there, it may lead to some champagne on holidays. It can be taken further to include extremely small amounts of other drinks. However it develops, this introduction to alcohol will help teach the child that it is acceptable to drink in certain situations, in appropriate amounts.
But friends and peers can help as well. They can encourage their friends to think about their actions and to abstain from excessive, inappropriate, irresponsible drinking. Peers have more of a voice than they think they do. Especially when they are concerned for their friends. If they think their friend isn't acting responsibly, they can always go talk to a parent or councilor about it. They may have to deal with their friend(s) being angry, but better they are angry than continuing on a path to alcoholism.
To finalize, alcohol abuse is an ever present occurrence. It can lead to severe physical, social, and legal consequences. But steps can, and should, be taken to prevent an increase in such behavior.
~Interminable Immediacy
Subscribe to:
Posts (Atom)